|
|
|

|
Suppliers for
Satraplatin
|
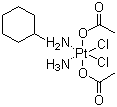
Properties | | CAS |
129580-63-8 | | Formula |
C10H22Cl2N2O4Pt |
|
|
9 Registered suppliers
Molecular Formula: C10H22Cl2N2O4Pt Molecular Weight: 500.28
More details are to be found here
More details are to be found here
Description : Satraplatin, also known as JM216, is a platinum compound that is currently under investigation as one treatment of patients with advanced prostate cancer who have failed previous chemotherapy. It has not yet received approval from the U.S. Food and Drug Administration. First mentioned in the medical literature in 1993, satraplatin is the first orally active platinum-based chemotherapeutic drug; other available platinum analogues-cisplatin, carboplatin, and oxaliplatin-must be given intravenously. It is made available in the United States jointly by Spectrum Pharmaceuticals and GPC Biotech under the name SPERA (Satraplatin Expanded Rapid Access). The drug has also been used in the treatment of lung and ovarian cancers. The proposed mode of action is that the compound binds to the DNA of cancer cells rendering them incapable of dividing. Molecular Formula : C10H24Cl2N2O4Pt Canonical SMILES : CC(=O)O.CC(=O)O.C1CCC(CC1)N.N.Cl[Pt]Cl InChI : InChI=1S/C6H13N.2C2H4O2.2ClH.H3N.Pt/c7-6-4-2-1-3-5-6;2*1-2(3)4;;;;/h6H,1-5,7H2;2*1H3,(H,3,4);2*1H;1H3;/q;;;;;;+2/p-2
InChIKey :
TWEQNPPRXJRHHM-UHFFFAOYSA-L
Synonyms :
BMS-182751; BMS 182751; BMS182751; JM 216; JM216; JM-216
More details are to be found here
Description : Satraplatin, also known as JM216, is a platinum compound that is currently under investigation as one treatment of patients with advanced prostate cancer who have failed previous chemotherapy. It has not yet received approval from the U.S. Food and Drug Administration. First mentioned in the medical literature in 1993, satraplatin is the first orally active platinum-based chemotherapeutic drug; other available platinum analogues-cisplatin, carboplatin, and oxaliplatin-must be given intravenously. It is made available in the United States jointly by Spectrum Pharmaceuticals and GPC Biotech under the name SPERA (Satraplatin Expanded Rapid Access). The drug has also been used in the treatment of lung and ovarian cancers. The proposed mode of action is that the compound binds to the DNA of cancer cells rendering them incapable of dividing. Molecular Formula : C10H24Cl2N2O4Pt Canonical SMILES : CC(=O)O.CC(=O)O.C1CCC(CC1)N.N.Cl[Pt]Cl InChI : InChI=1S/C6H13N.2C2H4O2.2ClH.H3N.Pt/c7-6-4-2-1-3-5-6;2*1-2(3)4;;;;/h6H,1-5,7H2;2*1H3,(H,3,4);2*1H;1H3;/q;;;;;;+2/p-2
InChIKey :
TWEQNPPRXJRHHM-UHFFFAOYSA-L
Synonyms :
BMS-182751; BMS 182751; BMS182751; JM 216; JM216; JM-216
More details are to be found here
Description : Satraplatin, also known as JM216, is a platinum compound that is currently under investigation as one treatment of patients with advanced prostate cancer who have failed previous chemotherapy. It has not yet received approval from the U.S. Food and Drug Administration. First mentioned in the medical literature in 1993, satraplatin is the first orally active platinum-based chemotherapeutic drug; other available platinum analogues-cisplatin, carboplatin, and oxaliplatin-must be given intravenously. It is made available in the United States jointly by Spectrum Pharmaceuticals and GPC Biotech under the name SPERA (Satraplatin Expanded Rapid Access). The drug has also been used in the treatment of lung and ovarian cancers. The proposed mode of action is that the compound binds to the DNA of cancer cells rendering them incapable of dividing. Molecular Formula : C10H24Cl2N2O4Pt Canonical SMILES : CC(=O)O.CC(=O)O.C1CCC(CC1)N.N.Cl[Pt]Cl InChI : InChI=1S/C6H13N.2C2H4O2.2ClH.H3N.Pt/c7-6-4-2-1-3-5-6;2*1-2(3)4;;;;/h6H,1-5,7H2;2*1H3,(H,3,4);2*1H;1H3;/q;;;;;;+2/p-2
InChIKey :
TWEQNPPRXJRHHM-UHFFFAOYSA-L
Synonyms :
BMS-182751; BMS 182751; BMS182751; JM 216; JM216; JM-216
More details are to be found here
Description : Satraplatin, also known as JM216, is a platinum compound that is currently under investigation as one treatment of patients with advanced prostate cancer who have failed previous chemotherapy. It has not yet received approval from the U.S. Food and Drug Administration. First mentioned in the medical literature in 1993, satraplatin is the first orally active platinum-based chemotherapeutic drug; other available platinum analogues-cisplatin, carboplatin, and oxaliplatin-must be given intravenously. It is made available in the United States jointly by Spectrum Pharmaceuticals and GPC Biotech under the name SPERA (Satraplatin Expanded Rapid Access). The drug has also been used in the treatment of lung and ovarian cancers. The proposed mode of action is that the compound binds to the DNA of cancer cells rendering them incapable of dividing. Molecular Formula : C10H24Cl2N2O4Pt Canonical SMILES : CC(=O)O.CC(=O)O.C1CCC(CC1)N.N.Cl[Pt]Cl InChI : InChI=1S/C6H13N.2C2H4O2.2ClH.H3N.Pt/c7-6-4-2-1-3-5-6;2*1-2(3)4;;;;/h6H,1-5,7H2;2*1H3,(H,3,4);2*1H;1H3;/q;;;;;;+2/p-2
InChIKey :
TWEQNPPRXJRHHM-UHFFFAOYSA-L
Synonyms :
BMS-182751; BMS 182751; BMS182751; JM 216; JM216; JM-216
More details are to be found here
Description : Satraplatin, also known as JM216, is a platinum compound that is currently under investigation as one treatment of patients with advanced prostate cancer who have failed previous chemotherapy. It has not yet received approval from the U.S. Food and Drug Administration. First mentioned in the medical literature in 1993, satraplatin is the first orally active platinum-based chemotherapeutic drug; other available platinum analogues-cisplatin, carboplatin, and oxaliplatin-must be given intravenously. It is made available in the United States jointly by Spectrum Pharmaceuticals and GPC Biotech under the name SPERA (Satraplatin Expanded Rapid Access). The drug has also been used in the treatment of lung and ovarian cancers. The proposed mode of action is that the compound binds to the DNA of cancer cells rendering them incapable of dividing. Molecular Formula : C10H24Cl2N2O4Pt Canonical SMILES : CC(=O)O.CC(=O)O.C1CCC(CC1)N.N.Cl[Pt]Cl InChI : InChI=1S/C6H13N.2C2H4O2.2ClH.H3N.Pt/c7-6-4-2-1-3-5-6;2*1-2(3)4;;;;/h6H,1-5,7H2;2*1H3,(H,3,4);2*1H;1H3;/q;;;;;;+2/p-2
InChIKey :
TWEQNPPRXJRHHM-UHFFFAOYSA-L
Synonyms :
BMS-182751; BMS 182751; BMS182751; JM 216; JM216; JM-216
More details are to be found here
|
Properties:
... more properties and specification on Satraplatin
|
|
Privileged suppliers
Last update 2024-04-26
|


 details
details details
details
_Co_Ltd_1_575.jpg)





