|
Suppliers for CAS
7772-99-8
|
Properties | | CAS |
7772-99-8 | | Formula |
SnCl2 | | EINECS |
231-868-0 |
|
29 Registered suppliers
Simagchem Corporation
P.R.China
Xiamen Hisunny Chemical Co., Ltd.
P.R.China
Molecular Formula : SnCl2
Dayang Chem (Hangzhou) Co.,Ltd.
P.R.China
Molecular Formula : SnCl2
Sancai Industry Co.,Ltd.
P.R.China
Molecular Formula : SnCl2
Xingrui Industry Co., Limited
P.R.China
Molecular Formula : SnCl2
Leap Chem Co., Ltd
P.R.China
Molecular Formula: Cl2Sn Molecular Weight: 189.62 Hazard Symbols: C,Xi,N UN Number: UN 3260 8/PG 3 WGKGermany: 1 PackingGroup: II HS Code: 28273990
More details are to be found here
Shanghai Mintchem Development Co., Ltd.
P.R.China
Molecular Formula: Cl2Sn Molecular Weight: 189.62 Hazard Symbols: C,Xi,N UN Number: UN 3260 8/PG 3 WGKGermany: 1 PackingGroup: II HS Code: 28273990
More details are to be found here
Alchimica s.r.o.
Czech Republic
Molecular Formula: Cl2Sn Molecular Weight: 189.62 Hazard Symbols: C,Xi,N UN Number: UN 3260 8/PG 3 WGKGermany: 1 PackingGroup: II HS Code: 28273990
More details are to be found here
VMP Chemiekontor GmbH
Germany
Molecular Formula: Cl2Sn Molecular Weight: 189.62 Hazard Symbols: C,Xi,N UN Number: UN 3260 8/PG 3 WGKGermany: 1 PackingGroup: II HS Code: 28273990
More details are to be found here
ProChem, Inc.
USA
Molecular Formula: Cl2Sn Molecular Weight: 189.62 Hazard Symbols: C,Xi,N UN Number: UN 3260 8/PG 3 WGKGermany: 1 PackingGroup: II HS Code: 28273990
More details are to be found here
Refine Chemical Co.,Ltd.
P.R.China
Molecular Formula: Cl2Sn Molecular Weight: 189.62 Hazard Symbols: C,Xi,N UN Number: UN 3260 8/PG 3 WGKGermany: 1 PackingGroup: II HS Code: 28273990
More details are to be found here
Unilong Industry Co.,Ltd
P.R.China
Molecular Formula: Cl2Sn Molecular Weight: 189.62 Hazard Symbols: C,Xi,N UN Number: UN 3260 8/PG 3 WGKGermany: 1 PackingGroup: II HS Code: 28273990
More details are to be found here
Wuhan PharmChem Co., LTD.
P.R.China
EINECS :231-868-0
Molecular formula :Cl2Sn
OPQ Chemical Co., Ltd
P.R.China
EINECS :231-868-0
Molecular formula :Cl2Sn
Create Chemical Co., Ltd.
P.R.China
| Molecular Fomula | Cl2Sn
| | Molecular Weight | 189.62
| | Appearance | White powder
| | Content | 99% |
Anderson & Steinssen Inc
USA
| Molecular Fomula | Cl2Sn
| | Molecular Weight | 189.62
| | Appearance | White powder
| | Content | 99% |
Shandong Lanhai Industry Co.,Ltd.
P.R.China
| Molecular Fomula | Cl2Sn
| | Molecular Weight | 189.62
| | Appearance | White powder
| | Content | 99% |
Shandong Minglang Chemical Co., Ltd
P.R.China
| Molecular Fomula | Cl2Sn
| | Molecular Weight | 189.62
| | Appearance | White powder
| | Content | 99% |
Fox Chemicals GmbH
Germany
| Molecular Fomula | Cl2Sn
| | Molecular Weight | 189.62
| | Appearance | White powder
| | Content | 99% |
Lori Industry Co., Ltd
P.R.China
| Molecular Fomula | Cl2Sn
| | Molecular Weight | 189.62
| | Appearance | White powder
| | Content | 99% |
Zehao Industry Co., Ltd.
P.R.China
| Molecular Fomula | Cl2Sn
| | Molecular Weight | 189.62
| | Appearance | White powder
| | Content | 99% |
Shandong SanYoung Industry Co., Ltd
P.R.China
| Molecular Fomula | Cl2Sn
| | Molecular Weight | 189.62
| | Appearance | White powder
| | Content | 99% |
Carbone Scientific Co., Ltd.
U.K.
| Molecular Fomula | Cl2Sn
| | Molecular Weight | 189.62
| | Appearance | White powder
| | Content | 99% |
BuGuCh & Partners
Germany
STANNOUS CHLORIDE Basic information
Product Name: STANNOUS CHLORIDE
Synonyms: Anhydrous stannous chloride;C.I. 77864;c.i.77864;chlorured’etain(ii);ci77864;dichlorotin;Fascat 2004;NCI-C02722
CAS: 7772-99-8
MF: Cl2Sn
MW: 189.62
EINECS: 231-868-0
Product Categories: Inorganic Chemicals;Inorganics;metal halide;Crystal Grade Inorganics;Tin Salts;Catalysis and Inorganic Chemistry;Chemical Synthesis;Materials Science;Metal and Ceramic Science;Salts;Tin
Mol File: 7772-99-8.mol
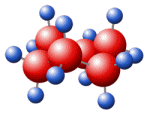
STANNOUS CHLORIDE Structure
STANNOUS CHLORIDE Chemical Properties
mp 246 °C(lit.)
bp 652 °C(lit.)
density 3.95
Fp 652°C
storage temp. Store at RT.
solubility H2O: soluble
form powder
Sensitive Air Sensitive & Hygroscopic
Merck 14,8783
Stability: Stable, but moisture sensitive. Incompatible with strong bases, strong oxidizing agents, reactive metals, hydrogen peroxide, water.
CAS DataBase Reference 7772-99-8(CAS DataBase Reference)
Safety Information
Hazard Codes C,Xi,N
Risk Statements 22-34-36/37/38-37-68-50/53-48/22-43-20-63
Safety Statements 26-36/37/39-45-61-60-53
RIDADR UN 3260 8/PG 3
WGK Germany 1
RTECS XP8700000
TSCA Yes
HazardClass 8
PackingGroup II
STANNOUS CHLORIDE Usage And Synthesis
Chemical Properties white crystalline solid
Usage Synthesis of tin(IV) octaethylcorroles was accomplished with this reagent. These new compounds exhibit reversible oxidation only at the conjugated ring system, not at the metal center.1
General Description Crystalline mass or flaky solid with a fatty appearance. Density 3.95 g / cm3. Melting point 247°C. Burns, but may be difficult to ignite. Toxic by ingestion. Irritates skin and eyes. Used in the manufacture of dyes, pharmaceuticals and as a tanning agent.
Air & Water Reactions Water soluble.
Reactivity Profile STANNOUS CHLORIDE is a powerful reducing agent. Can react violently with oxidizing agents. Undergoes flaming reaction with bromine trifluoride [Mellor 2 Supp. 1:164 1956]. Catalyzes the exothermic rearrangement and polymerization of ethylene oxide [J. Soc. Chem. Ind. 68:179 1949]. Mixtures with calcium carbide can be ignited with a match, and the reaction proceeds with incandescence [Mellor 7:430 1946-47]. Reacts with hydrazine hydrate to give stannous dihydrazine chloride which decomposes explosively when heated [Mellor 7:430 1946-47]. Undergoes a strongly exothermic reaction with aqueous solutions of hydrogen peroxide having concentration exceeding 3%) [Chem. & Ind., 1949, 657].
Health Hazard TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
STANNOUS CHLORIDE Basic information
Product Name: STANNOUS CHLORIDE
Synonyms: Anhydrous stannous chloride;C.I. 77864;c.i.77864;chlorured’etain(ii);ci77864;dichlorotin;Fascat 2004;NCI-C02722
CAS: 7772-99-8
MF: Cl2Sn
MW: 189.62
EINECS: 231-868-0
Product Categories: Inorganic Chemicals;Inorganics;metal halide;Crystal Grade Inorganics;Tin Salts;Catalysis and Inorganic Chemistry;Chemical Synthesis;Materials Science;Metal and Ceramic Science;Salts;Tin
Mol File: 7772-99-8.mol
STANNOUS CHLORIDE Structure
STANNOUS CHLORIDE Chemical Properties
mp 246 °C(lit.)
bp 652 °C(lit.)
density 3.95
Fp 652°C
storage temp. Store at RT.
solubility H2O: soluble
form powder
Sensitive Air Sensitive & Hygroscopic
Merck 14,8783
Stability: Stable, but moisture sensitive. Incompatible with strong bases, strong oxidizing agents, reactive metals, hydrogen peroxide, water.
CAS DataBase Reference 7772-99-8(CAS DataBase Reference)
Safety Information
Hazard Codes C,Xi,N
Risk Statements 22-34-36/37/38-37-68-50/53-48/22-43-20-63
Safety Statements 26-36/37/39-45-61-60-53
RIDADR UN 3260 8/PG 3
WGK Germany 1
RTECS XP8700000
TSCA Yes
HazardClass 8
PackingGroup II
STANNOUS CHLORIDE Usage And Synthesis
Chemical Properties white crystalline solid
Usage Synthesis of tin(IV) octaethylcorroles was accomplished with this reagent. These new compounds exhibit reversible oxidation only at the conjugated ring system, not at the metal center.1
General Description Crystalline mass or flaky solid with a fatty appearance. Density 3.95 g / cm3. Melting point 247°C. Burns, but may be difficult to ignite. Toxic by ingestion. Irritates skin and eyes. Used in the manufacture of dyes, pharmaceuticals and as a tanning agent.
Air & Water Reactions Water soluble.
Reactivity Profile STANNOUS CHLORIDE is a powerful reducing agent. Can react violently with oxidizing agents. Undergoes flaming reaction with bromine trifluoride [Mellor 2 Supp. 1:164 1956]. Catalyzes the exothermic rearrangement and polymerization of ethylene oxide [J. Soc. Chem. Ind. 68:179 1949]. Mixtures with calcium carbide can be ignited with a match, and the reaction proceeds with incandescence [Mellor 7:430 1946-47]. Reacts with hydrazine hydrate to give stannous dihydrazine chloride which decomposes explosively when heated [Mellor 7:430 1946-47]. Undergoes a strongly exothermic reaction with aqueous solutions of hydrogen peroxide having concentration exceeding 3%) [Chem. & Ind., 1949, 657].
Health Hazard TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
William Blythe Ltd.
U.K.
STANNOUS CHLORIDE Basic information
Product Name: STANNOUS CHLORIDE
Synonyms: Anhydrous stannous chloride;C.I. 77864;c.i.77864;chlorured’etain(ii);ci77864;dichlorotin;Fascat 2004;NCI-C02722
CAS: 7772-99-8
MF: Cl2Sn
MW: 189.62
EINECS: 231-868-0
Product Categories: Inorganic Chemicals;Inorganics;metal halide;Crystal Grade Inorganics;Tin Salts;Catalysis and Inorganic Chemistry;Chemical Synthesis;Materials Science;Metal and Ceramic Science;Salts;Tin
Mol File: 7772-99-8.mol
STANNOUS CHLORIDE Structure
STANNOUS CHLORIDE Chemical Properties
mp 246 °C(lit.)
bp 652 °C(lit.)
density 3.95
Fp 652°C
storage temp. Store at RT.
solubility H2O: soluble
form powder
Sensitive Air Sensitive & Hygroscopic
Merck 14,8783
Stability: Stable, but moisture sensitive. Incompatible with strong bases, strong oxidizing agents, reactive metals, hydrogen peroxide, water.
CAS DataBase Reference 7772-99-8(CAS DataBase Reference)
Safety Information
Hazard Codes C,Xi,N
Risk Statements 22-34-36/37/38-37-68-50/53-48/22-43-20-63
Safety Statements 26-36/37/39-45-61-60-53
RIDADR UN 3260 8/PG 3
WGK Germany 1
RTECS XP8700000
TSCA Yes
HazardClass 8
PackingGroup II
STANNOUS CHLORIDE Usage And Synthesis
Chemical Properties white crystalline solid
Usage Synthesis of tin(IV) octaethylcorroles was accomplished with this reagent. These new compounds exhibit reversible oxidation only at the conjugated ring system, not at the metal center.1
General Description Crystalline mass or flaky solid with a fatty appearance. Density 3.95 g / cm3. Melting point 247°C. Burns, but may be difficult to ignite. Toxic by ingestion. Irritates skin and eyes. Used in the manufacture of dyes, pharmaceuticals and as a tanning agent.
Air & Water Reactions Water soluble.
Reactivity Profile STANNOUS CHLORIDE is a powerful reducing agent. Can react violently with oxidizing agents. Undergoes flaming reaction with bromine trifluoride [Mellor 2 Supp. 1:164 1956]. Catalyzes the exothermic rearrangement and polymerization of ethylene oxide [J. Soc. Chem. Ind. 68:179 1949]. Mixtures with calcium carbide can be ignited with a match, and the reaction proceeds with incandescence [Mellor 7:430 1946-47]. Reacts with hydrazine hydrate to give stannous dihydrazine chloride which decomposes explosively when heated [Mellor 7:430 1946-47]. Undergoes a strongly exothermic reaction with aqueous solutions of hydrogen peroxide having concentration exceeding 3%) [Chem. & Ind., 1949, 657].
Health Hazard TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
STANNOUS CHLORIDE Basic information
Product Name: STANNOUS CHLORIDE
Synonyms: Anhydrous stannous chloride;C.I. 77864;c.i.77864;chlorured’etain(ii);ci77864;dichlorotin;Fascat 2004;NCI-C02722
CAS: 7772-99-8
MF: Cl2Sn
MW: 189.62
EINECS: 231-868-0
Product Categories: Inorganic Chemicals;Inorganics;metal halide;Crystal Grade Inorganics;Tin Salts;Catalysis and Inorganic Chemistry;Chemical Synthesis;Materials Science;Metal and Ceramic Science;Salts;Tin
Mol File: 7772-99-8.mol
STANNOUS CHLORIDE Structure
STANNOUS CHLORIDE Chemical Properties
mp 246 °C(lit.)
bp 652 °C(lit.)
density 3.95
Fp 652°C
storage temp. Store at RT.
solubility H2O: soluble
form powder
Sensitive Air Sensitive & Hygroscopic
Merck 14,8783
Stability: Stable, but moisture sensitive. Incompatible with strong bases, strong oxidizing agents, reactive metals, hydrogen peroxide, water.
CAS DataBase Reference 7772-99-8(CAS DataBase Reference)
Safety Information
Hazard Codes C,Xi,N
Risk Statements 22-34-36/37/38-37-68-50/53-48/22-43-20-63
Safety Statements 26-36/37/39-45-61-60-53
RIDADR UN 3260 8/PG 3
WGK Germany 1
RTECS XP8700000
TSCA Yes
HazardClass 8
PackingGroup II
STANNOUS CHLORIDE Usage And Synthesis
Chemical Properties white crystalline solid
Usage Synthesis of tin(IV) octaethylcorroles was accomplished with this reagent. These new compounds exhibit reversible oxidation only at the conjugated ring system, not at the metal center.1
General Description Crystalline mass or flaky solid with a fatty appearance. Density 3.95 g / cm3. Melting point 247°C. Burns, but may be difficult to ignite. Toxic by ingestion. Irritates skin and eyes. Used in the manufacture of dyes, pharmaceuticals and as a tanning agent.
Air & Water Reactions Water soluble.
Reactivity Profile STANNOUS CHLORIDE is a powerful reducing agent. Can react violently with oxidizing agents. Undergoes flaming reaction with bromine trifluoride [Mellor 2 Supp. 1:164 1956]. Catalyzes the exothermic rearrangement and polymerization of ethylene oxide [J. Soc. Chem. Ind. 68:179 1949]. Mixtures with calcium carbide can be ignited with a match, and the reaction proceeds with incandescence [Mellor 7:430 1946-47]. Reacts with hydrazine hydrate to give stannous dihydrazine chloride which decomposes explosively when heated [Mellor 7:430 1946-47]. Undergoes a strongly exothermic reaction with aqueous solutions of hydrogen peroxide having concentration exceeding 3%) [Chem. & Ind., 1949, 657].
Health Hazard TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Avonchem Ltd.
U.K.
STANNOUS CHLORIDE Basic information
Product Name: STANNOUS CHLORIDE
Synonyms: Anhydrous stannous chloride;C.I. 77864;c.i.77864;chlorured’etain(ii);ci77864;dichlorotin;Fascat 2004;NCI-C02722
CAS: 7772-99-8
MF: Cl2Sn
MW: 189.62
EINECS: 231-868-0
Product Categories: Inorganic Chemicals;Inorganics;metal halide;Crystal Grade Inorganics;Tin Salts;Catalysis and Inorganic Chemistry;Chemical Synthesis;Materials Science;Metal and Ceramic Science;Salts;Tin
Mol File: 7772-99-8.mol
STANNOUS CHLORIDE Structure
STANNOUS CHLORIDE Chemical Properties
mp 246 °C(lit.)
bp 652 °C(lit.)
density 3.95
Fp 652°C
storage temp. Store at RT.
solubility H2O: soluble
form powder
Sensitive Air Sensitive & Hygroscopic
Merck 14,8783
Stability: Stable, but moisture sensitive. Incompatible with strong bases, strong oxidizing agents, reactive metals, hydrogen peroxide, water.
CAS DataBase Reference 7772-99-8(CAS DataBase Reference)
Safety Information
Hazard Codes C,Xi,N
Risk Statements 22-34-36/37/38-37-68-50/53-48/22-43-20-63
Safety Statements 26-36/37/39-45-61-60-53
RIDADR UN 3260 8/PG 3
WGK Germany 1
RTECS XP8700000
TSCA Yes
HazardClass 8
PackingGroup II
STANNOUS CHLORIDE Usage And Synthesis
Chemical Properties white crystalline solid
Usage Synthesis of tin(IV) octaethylcorroles was accomplished with this reagent. These new compounds exhibit reversible oxidation only at the conjugated ring system, not at the metal center.1
General Description Crystalline mass or flaky solid with a fatty appearance. Density 3.95 g / cm3. Melting point 247°C. Burns, but may be difficult to ignite. Toxic by ingestion. Irritates skin and eyes. Used in the manufacture of dyes, pharmaceuticals and as a tanning agent.
Air & Water Reactions Water soluble.
Reactivity Profile STANNOUS CHLORIDE is a powerful reducing agent. Can react violently with oxidizing agents. Undergoes flaming reaction with bromine trifluoride [Mellor 2 Supp. 1:164 1956]. Catalyzes the exothermic rearrangement and polymerization of ethylene oxide [J. Soc. Chem. Ind. 68:179 1949]. Mixtures with calcium carbide can be ignited with a match, and the reaction proceeds with incandescence [Mellor 7:430 1946-47]. Reacts with hydrazine hydrate to give stannous dihydrazine chloride which decomposes explosively when heated [Mellor 7:430 1946-47]. Undergoes a strongly exothermic reaction with aqueous solutions of hydrogen peroxide having concentration exceeding 3%) [Chem. & Ind., 1949, 657].
Health Hazard TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Merck Schuchardt OHG
Germany
STANNOUS CHLORIDE Basic information
Product Name: STANNOUS CHLORIDE
Synonyms: Anhydrous stannous chloride;C.I. 77864;c.i.77864;chlorured’etain(ii);ci77864;dichlorotin;Fascat 2004;NCI-C02722
CAS: 7772-99-8
MF: Cl2Sn
MW: 189.62
EINECS: 231-868-0
Product Categories: Inorganic Chemicals;Inorganics;metal halide;Crystal Grade Inorganics;Tin Salts;Catalysis and Inorganic Chemistry;Chemical Synthesis;Materials Science;Metal and Ceramic Science;Salts;Tin
Mol File: 7772-99-8.mol
STANNOUS CHLORIDE Structure
STANNOUS CHLORIDE Chemical Properties
mp 246 °C(lit.)
bp 652 °C(lit.)
density 3.95
Fp 652°C
storage temp. Store at RT.
solubility H2O: soluble
form powder
Sensitive Air Sensitive & Hygroscopic
Merck 14,8783
Stability: Stable, but moisture sensitive. Incompatible with strong bases, strong oxidizing agents, reactive metals, hydrogen peroxide, water.
CAS DataBase Reference 7772-99-8(CAS DataBase Reference)
Safety Information
Hazard Codes C,Xi,N
Risk Statements 22-34-36/37/38-37-68-50/53-48/22-43-20-63
Safety Statements 26-36/37/39-45-61-60-53
RIDADR UN 3260 8/PG 3
WGK Germany 1
RTECS XP8700000
TSCA Yes
HazardClass 8
PackingGroup II
STANNOUS CHLORIDE Usage And Synthesis
Chemical Properties white crystalline solid
Usage Synthesis of tin(IV) octaethylcorroles was accomplished with this reagent. These new compounds exhibit reversible oxidation only at the conjugated ring system, not at the metal center.1
General Description Crystalline mass or flaky solid with a fatty appearance. Density 3.95 g / cm3. Melting point 247°C. Burns, but may be difficult to ignite. Toxic by ingestion. Irritates skin and eyes. Used in the manufacture of dyes, pharmaceuticals and as a tanning agent.
Air & Water Reactions Water soluble.
Reactivity Profile STANNOUS CHLORIDE is a powerful reducing agent. Can react violently with oxidizing agents. Undergoes flaming reaction with bromine trifluoride [Mellor 2 Supp. 1:164 1956]. Catalyzes the exothermic rearrangement and polymerization of ethylene oxide [J. Soc. Chem. Ind. 68:179 1949]. Mixtures with calcium carbide can be ignited with a match, and the reaction proceeds with incandescence [Mellor 7:430 1946-47]. Reacts with hydrazine hydrate to give stannous dihydrazine chloride which decomposes explosively when heated [Mellor 7:430 1946-47]. Undergoes a strongly exothermic reaction with aqueous solutions of hydrogen peroxide having concentration exceeding 3%) [Chem. & Ind., 1949, 657].
Health Hazard TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
CM Fine Chemicals
Switzerland
STANNOUS CHLORIDE Basic information
Product Name: STANNOUS CHLORIDE
Synonyms: Anhydrous stannous chloride;C.I. 77864;c.i.77864;chlorured’etain(ii);ci77864;dichlorotin;Fascat 2004;NCI-C02722
CAS: 7772-99-8
MF: Cl2Sn
MW: 189.62
EINECS: 231-868-0
Product Categories: Inorganic Chemicals;Inorganics;metal halide;Crystal Grade Inorganics;Tin Salts;Catalysis and Inorganic Chemistry;Chemical Synthesis;Materials Science;Metal and Ceramic Science;Salts;Tin
Mol File: 7772-99-8.mol
STANNOUS CHLORIDE Structure
STANNOUS CHLORIDE Chemical Properties
mp 246 °C(lit.)
bp 652 °C(lit.)
density 3.95
Fp 652°C
storage temp. Store at RT.
solubility H2O: soluble
form powder
Sensitive Air Sensitive & Hygroscopic
Merck 14,8783
Stability: Stable, but moisture sensitive. Incompatible with strong bases, strong oxidizing agents, reactive metals, hydrogen peroxide, water.
CAS DataBase Reference 7772-99-8(CAS DataBase Reference)
Safety Information
Hazard Codes C,Xi,N
Risk Statements 22-34-36/37/38-37-68-50/53-48/22-43-20-63
Safety Statements 26-36/37/39-45-61-60-53
RIDADR UN 3260 8/PG 3
WGK Germany 1
RTECS XP8700000
TSCA Yes
HazardClass 8
PackingGroup II
STANNOUS CHLORIDE Usage And Synthesis
Chemical Properties white crystalline solid
Usage Synthesis of tin(IV) octaethylcorroles was accomplished with this reagent. These new compounds exhibit reversible oxidation only at the conjugated ring system, not at the metal center.1
General Description Crystalline mass or flaky solid with a fatty appearance. Density 3.95 g / cm3. Melting point 247°C. Burns, but may be difficult to ignite. Toxic by ingestion. Irritates skin and eyes. Used in the manufacture of dyes, pharmaceuticals and as a tanning agent.
Air & Water Reactions Water soluble.
Reactivity Profile STANNOUS CHLORIDE is a powerful reducing agent. Can react violently with oxidizing agents. Undergoes flaming reaction with bromine trifluoride [Mellor 2 Supp. 1:164 1956]. Catalyzes the exothermic rearrangement and polymerization of ethylene oxide [J. Soc. Chem. Ind. 68:179 1949]. Mixtures with calcium carbide can be ignited with a match, and the reaction proceeds with incandescence [Mellor 7:430 1946-47]. Reacts with hydrazine hydrate to give stannous dihydrazine chloride which decomposes explosively when heated [Mellor 7:430 1946-47]. Undergoes a strongly exothermic reaction with aqueous solutions of hydrogen peroxide having concentration exceeding 3%) [Chem. & Ind., 1949, 657].
Health Hazard TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Santa Cruz Biotechnology, Inc.
USA
STANNOUS CHLORIDE Basic information
Product Name: STANNOUS CHLORIDE
Synonyms: Anhydrous stannous chloride;C.I. 77864;c.i.77864;chlorured’etain(ii);ci77864;dichlorotin;Fascat 2004;NCI-C02722
CAS: 7772-99-8
MF: Cl2Sn
MW: 189.62
EINECS: 231-868-0
Product Categories: Inorganic Chemicals;Inorganics;metal halide;Crystal Grade Inorganics;Tin Salts;Catalysis and Inorganic Chemistry;Chemical Synthesis;Materials Science;Metal and Ceramic Science;Salts;Tin
Mol File: 7772-99-8.mol
STANNOUS CHLORIDE Structure
STANNOUS CHLORIDE Chemical Properties
mp 246 °C(lit.)
bp 652 °C(lit.)
density 3.95
Fp 652°C
storage temp. Store at RT.
solubility H2O: soluble
form powder
Sensitive Air Sensitive & Hygroscopic
Merck 14,8783
Stability: Stable, but moisture sensitive. Incompatible with strong bases, strong oxidizing agents, reactive metals, hydrogen peroxide, water.
CAS DataBase Reference 7772-99-8(CAS DataBase Reference)
Safety Information
Hazard Codes C,Xi,N
Risk Statements 22-34-36/37/38-37-68-50/53-48/22-43-20-63
Safety Statements 26-36/37/39-45-61-60-53
RIDADR UN 3260 8/PG 3
WGK Germany 1
RTECS XP8700000
TSCA Yes
HazardClass 8
PackingGroup II
STANNOUS CHLORIDE Usage And Synthesis
Chemical Properties white crystalline solid
Usage Synthesis of tin(IV) octaethylcorroles was accomplished with this reagent. These new compounds exhibit reversible oxidation only at the conjugated ring system, not at the metal center.1
General Description Crystalline mass or flaky solid with a fatty appearance. Density 3.95 g / cm3. Melting point 247°C. Burns, but may be difficult to ignite. Toxic by ingestion. Irritates skin and eyes. Used in the manufacture of dyes, pharmaceuticals and as a tanning agent.
Air & Water Reactions Water soluble.
Reactivity Profile STANNOUS CHLORIDE is a powerful reducing agent. Can react violently with oxidizing agents. Undergoes flaming reaction with bromine trifluoride [Mellor 2 Supp. 1:164 1956]. Catalyzes the exothermic rearrangement and polymerization of ethylene oxide [J. Soc. Chem. Ind. 68:179 1949]. Mixtures with calcium carbide can be ignited with a match, and the reaction proceeds with incandescence [Mellor 7:430 1946-47]. Reacts with hydrazine hydrate to give stannous dihydrazine chloride which decomposes explosively when heated [Mellor 7:430 1946-47]. Undergoes a strongly exothermic reaction with aqueous solutions of hydrogen peroxide having concentration exceeding 3%) [Chem. & Ind., 1949, 657].
Health Hazard TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
|

 details
details details
details details
details

_Co_Ltd_1_575.jpg)






