|
|
|

|
Suppliers for
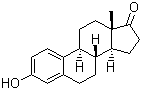
Estrone
|
Properties | | CAS |
53-16-7 | | Formula |
C18H22O2 | | EINECS |
200-164-5 |
|
|
19 Registered suppliers
Molecular Formula: C18H22O2 Molecular Weight: 270.37
More details are to be found here
Molecular Formula: C18H22O2 Molecular Weight: 270.37
More details are to be found here
Molecular Formula: C18H22O2 Molecular Weight: 270.37
More details are to be found here
EINECS :200-164-5
Molecular formula :C18H22O2
EINECS :200-164-5
Molecular formula :C18H22O2
EINECS :200-164-5
Molecular formula :C18H22O2
EINECS :200-164-5
Molecular formula :C18H22O2
EINECS :200-164-5
Molecular formula :C18H22O2
EINECS :200-164-5
Molecular formula :C18H22O2
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Detailed information on the suppliers of
Estrone |
Properties:
... more properties and specification on Estrone
|
|
Privileged suppliers
Last update 2024-04-29
|


 details
details details
details








