|
|
|

|
Suppliers for
Sodium methylate (solution 30% in methanol)
|
Properties | | CAS |
124-41-4 | | Formula |
CH3ONa | | EINECS |
204-699-5 |
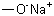
|
|
2 Registered suppliers
| Synonyms: | Methanol, sodium salt; Methanol,sodiumsalt; methoxysodium;
metilatosodico; sodiummethoxide, 30%(ww)solution in methanol
| | CAS: | 124-41-4
| | MF: | CH3NaO
| | MW: | 54.02
| | EINECS: | 204-699-5
| | Product Categories: | Organics;Classes of Metal Compounds;Na (Sodium) Compounds (excluding simple sodium salts);Typical Metal Compounds;metal alkoxide
| | Mol File: | 124-41-4.mol
| | mp : | -98 °C
| | bp : | 65 °C
| | density: | 0.97 g/mL at 20 °C
| | vapor density : | 1.1 (vs air)
| | vapor pressure : | 50 mm Hg ( 20 °C)
| | refractive index : | 1.3700
| | Fp : | 11 °C
| | storage temp. : | Flammables area
| | Water Solubility : | reacts
| | Sensitive : | Moisture Sensitive
| | Merck : | 14,8643
| | BRN : | 3592982
| | Stability: | Stability Highly flammable. Reacts violently with water. Keep container dry. Incompatible with water, acids, chlorinated solvents.
| | NIST: | Chemistry Reference Sodium methoxide(124-41-4)
| | Hazard Codes : | F,T,C
| | Risk Statements : | 11-23/24/25-34-39/23/24/25-36/38-14-36/37/38
| | Safety Statements : | 8-16-26-43-45-7-36/37-7/8-36/37/39
| | RIDADR: | UN 1431 4.2/PG 2
| | WGK Germany : | 1
| | F : | 9-21
| | TSCA : | Yes
| | HazardClass : | 4.2
| | PackingGroup : | II
| | Hazardous Substances Data: | 124-41-4(Hazardous Substances Data)
| General Description A colorless cloudy white liquid consisting of sodium methylate, a solid, dissolved in methyl alcohol. Density 9.0 lb / gal. Corrosive to metals and tissue. Used to process edible fats and oils and to make other chemicals.
| Air & Water Reactions Highly flammable. Ignites in moist air [Wischmeyer 1966]. Reacts with water to produce a mixed solution of sodium hydroxide and methyl alcohol.
| Reactivity Profile SODIUM METHYLATE is a strong base. Reacts with light metals forming H2 gas, with fire and explosion hazards. Too rapid addition of sodium methylate to a mixture of chloroform and methanol initiated an uncontrolled exothermic reaction between the chloroform and the methylate that caused a violent explosion [MCA Case History 693 1961]. Sodium methoxide is incompatible with 4-chloronitrobenzene and fluorinated cyclopropenyl methyl ethers, such as perfluoromethoxycyclopropene. The reactions are vigorous and may initiate ignition [Bretherick, 1995, pg. 191].
| Health Hazard TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
| | Fire Hazard Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. |
| Synonyms: | Methanol, sodium salt; Methanol,sodiumsalt; methoxysodium;
metilatosodico; sodiummethoxide, 30%(ww)solution in methanol
| | CAS: | 124-41-4
| | MF: | CH3NaO
| | MW: | 54.02
| | EINECS: | 204-699-5
| | Product Categories: | Organics;Classes of Metal Compounds;Na (Sodium) Compounds (excluding simple sodium salts);Typical Metal Compounds;metal alkoxide
| | Mol File: | 124-41-4.mol
| | mp : | -98 °C
| | bp : | 65 °C
| | density: | 0.97 g/mL at 20 °C
| | vapor density : | 1.1 (vs air)
| | vapor pressure : | 50 mm Hg ( 20 °C)
| | refractive index : | 1.3700
| | Fp : | 11 °C
| | storage temp. : | Flammables area
| | Water Solubility : | reacts
| | Sensitive : | Moisture Sensitive
| | Merck : | 14,8643
| | BRN : | 3592982
| | Stability: | Stability Highly flammable. Reacts violently with water. Keep container dry. Incompatible with water, acids, chlorinated solvents.
| | NIST: | Chemistry Reference Sodium methoxide(124-41-4)
| | Hazard Codes : | F,T,C
| | Risk Statements : | 11-23/24/25-34-39/23/24/25-36/38-14-36/37/38
| | Safety Statements : | 8-16-26-43-45-7-36/37-7/8-36/37/39
| | RIDADR: | UN 1431 4.2/PG 2
| | WGK Germany : | 1
| | F : | 9-21
| | TSCA : | Yes
| | HazardClass : | 4.2
| | PackingGroup : | II
| | Hazardous Substances Data: | 124-41-4(Hazardous Substances Data)
| General Description A colorless cloudy white liquid consisting of sodium methylate, a solid, dissolved in methyl alcohol. Density 9.0 lb / gal. Corrosive to metals and tissue. Used to process edible fats and oils and to make other chemicals.
| Air & Water Reactions Highly flammable. Ignites in moist air [Wischmeyer 1966]. Reacts with water to produce a mixed solution of sodium hydroxide and methyl alcohol.
| Reactivity Profile SODIUM METHYLATE is a strong base. Reacts with light metals forming H2 gas, with fire and explosion hazards. Too rapid addition of sodium methylate to a mixture of chloroform and methanol initiated an uncontrolled exothermic reaction between the chloroform and the methylate that caused a violent explosion [MCA Case History 693 1961]. Sodium methoxide is incompatible with 4-chloronitrobenzene and fluorinated cyclopropenyl methyl ethers, such as perfluoromethoxycyclopropene. The reactions are vigorous and may initiate ignition [Bretherick, 1995, pg. 191].
| Health Hazard TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
| | Fire Hazard Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. |
|
|
|
Privileged suppliers
Last update 2024-04-29
|

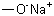
 details
details