|
Suppliers for CAS
56553-60-7
|
Properties | | CAS |
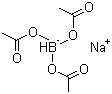
56553-60-7 | | Formula |
C6H10BNaO6 |
|
|
37 Registered suppliers
Simagchem Corporation
P.R.China
Xiamen Hisunny Chemical Co., Ltd.
P.R.China
Molecular Formula : C6H10BNaO6
Aecochem Corp.
P.R.China
Molecular Formula : C6H10BNaO6
Amadis Chemical Company Limited
P.R.China
Molecular Formula : C6H10BNaO6
Dayang Chem (Hangzhou) Co.,Ltd.
P.R.China
Molecular Formula : C6H10BNaO6
Sancai Industry Co.,Ltd.
P.R.China
| Appearance | White powder
| | Melting point | 114-118 ºC
| | Assay | 98% |
Hangzhou Keying Chem Co., Ltd.
P.R.China
| Appearance | White powder
| | Melting point | 114-118 ºC
| | Assay | 98% |
H&Z Industry Co.,Ltd
P.R.China
| Appearance | White powder
| | Melting point | 114-118 ºC
| | Assay | 98% |
Xingrui Industry Co., Limited
P.R.China
| Appearance | White powder
| | Melting point | 114-118 ºC
| | Assay | 98% |
Hangzhou Meite Industry Co., Ltd (Hangzhou Meite Chemical Co., Ltd)
P.R.China
| Appearance | White powder
| | Melting point | 114-118 ºC
| | Assay | 98% |
Capot Chemical Co., Ltd.
P.R.China
More details are to be found here
Leap Chem Co., Ltd
P.R.China
Molecular Formula: C6H10BNaO6 Molecular Weight: 211.94 Hazard Symbols: F,Xi,C UN Number: UN 1409 4.3/PG 2 WGKGermany: 3 PackingGroup: III HS Code: 29319090
More details are to be found here
BASF SE
Germany
Molecular Formula: C6H10BNaO6 Molecular Weight: 211.94 Hazard Symbols: F,Xi,C UN Number: UN 1409 4.3/PG 2 WGKGermany: 3 PackingGroup: III HS Code: 29319090
More details are to be found here
KHBoddin GmbH
Germany
Molecular Formula: C6H10BNaO6 Molecular Weight: 211.94 Hazard Symbols: F,Xi,C UN Number: UN 1409 4.3/PG 2 WGKGermany: 3 PackingGroup: III HS Code: 29319090
More details are to be found here
S. Goldmann GmbH & Co KG
Germany
Molecular Formula: C6H10BNaO6 Molecular Weight: 211.94 Hazard Symbols: F,Xi,C UN Number: UN 1409 4.3/PG 2 WGKGermany: 3 PackingGroup: III HS Code: 29319090
More details are to be found here
Hangzhou Verychem Science & Technology Co., Ltd.
P.R.China
Molecular Formula: C6H10BNaO6 Molecular Weight: 211.94 Hazard Symbols: F,Xi,C UN Number: UN 1409 4.3/PG 2 WGKGermany: 3 PackingGroup: III HS Code: 29319090
More details are to be found here
Shandong Ench Chemical Co.,Ltd
P.R.China
Molecular Formula: C6H10BNaO6 Molecular Weight: 211.94 Hazard Symbols: F,Xi,C UN Number: UN 1409 4.3/PG 2 WGKGermany: 3 PackingGroup: III HS Code: 29319090
More details are to be found here
JieJie Group Co., Ltd.
P.R.China
Molecular Formula: C6H10BNaO6 Molecular Weight: 211.94 Hazard Symbols: F,Xi,C UN Number: UN 1409 4.3/PG 2 WGKGermany: 3 PackingGroup: III HS Code: 29319090
More details are to be found here
Krada CPS Industry S.L
Spain
Molecular Formula: C6H10BNaO6 Molecular Weight: 211.94 Hazard Symbols: F,Xi,C UN Number: UN 1409 4.3/PG 2 WGKGermany: 3 PackingGroup: III HS Code: 29319090
More details are to be found here
Shandong Minglang Chemical Co., Ltd
P.R.China
Molecular Formula: C6H10BNaO6 Molecular Weight: 211.94 Hazard Symbols: F,Xi,C UN Number: UN 1409 4.3/PG 2 WGKGermany: 3 PackingGroup: III HS Code: 29319090
More details are to be found here
Hangzhou Zhongqi Chem Co., Ltd
P.R.China
Molecular Formula: C6H10BNaO6 Molecular Weight: 211.94 Hazard Symbols: F,Xi,C UN Number: UN 1409 4.3/PG 2 WGKGermany: 3 PackingGroup: III HS Code: 29319090
More details are to be found here
Nantong Xindao Biotech Ltd
P.R.China
Molecular Formula: C6H10BNaO6 Molecular Weight: 211.94 Hazard Symbols: F,Xi,C UN Number: UN 1409 4.3/PG 2 WGKGermany: 3 PackingGroup: III HS Code: 29319090
More details are to be found here
Lori Industry Co., Ltd
P.R.China
Molecular Formula: C6H10BNaO6 Molecular Weight: 211.94 Hazard Symbols: F,Xi,C UN Number: UN 1409 4.3/PG 2 WGKGermany: 3 PackingGroup: III HS Code: 29319090
More details are to be found here
Zehao Industry Co., Ltd.
P.R.China
Finetech Industry Limited
P.R.China
More details are to be found here
Hangzhou Lingrui Chemical Co., Ltd.
P.R.China
More details are to be found here
Skyrun Industrial Co., Ltd.
P.R.China
More details are to be found here
Shandong SanYoung Industry Co., Ltd
P.R.China
More details are to be found here
AK Scientific, Inc.
USA
More details are to be found here
Chemos GmbH & Co. KG
Germany
More details are to be found here
BuGuCh & Partners
Germany
Product name: Sodium triacetoxyborohydride
Synonyms:stab;SODIUM TRIACETOXYBOROHYDRIDE;SODIUM TRIACETOXYHYDROBORATE; sodiumtriacetoxyborohydride,calselect?stab;Sodium Triacetoxyborohyride;Sodium triacetoborohydride;Sodium triacetohydroborate, Triacetohydroborate sodium salt;Sodiumtriacetoxyborohydride,95%
CAS: 56553-60-7
Formula: C6H10BNaO6
Molecular weight: 211.94
Chemical properties: triacetoxy sodium boron hydride, a white crystalline powder; soluble in benzene and tetrahydrofuran, ketones and aldehydes used for the reductive amination reaction of carbonyl compounds and amines reductive amination / lactamization, and aryl aldehyde reductive amination.
Soluble in benzene and tetrahydrofuran. Is milder selective borohydride reagents. Reacts with water, dissolved in THF, acetonitrile and 1,2-dichloroethane.
Melting point: 116-120 °C (dec.)(lit.)
Storage condition:Flammables + water-Freezer (-20°C)e area
Water solubility:reacts
Sensitivity:Moisture Sensitive
Merck :8695
BRN :4047608
Usage:
1.The new catalyst for the reductive amination with excellent universality and selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification, the catalyst itself and the byproducts are non-toxic, no environmental pollution, become reductive amination catalyst of choice.
2.Ketones and aldehydes used for the reduction reaction of an amine, carbonyl compounds and amines reductive amination / amidation inside, and aryl aldehyde reductive amination.
3.Triacetoxy sodium borohydride is a new catalyst for the reductive amination developed in recent years, because of its universality and excellent selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification the catalyst itself and the byproducts are non-toxic, no environmental pollution, as a catalyst for reductive amination preferred.
4.Catalytic hydrogenation in the metal catalyst of platinum, palladium, nickel or the like, although the method is more economical and has applied to the advantage of mass production, but got more than a mixture, and lower yields, as well as limitations on the structure carbon-carbon multiple bond can be reduced and there are functional groups present in the compounds, such as those containing nitro, cyano, catalytic hydrogenation method can not be used, and the catalytic performance is often inhibited by divalent sulfides.
5.Reductive amination refers to the conversion of carbonyl compounds to amines important organic reactions. Imine formed first, then reduced with sodium borohydride; either sodium borohydride, or triacetoxy sodium borohydride, sodium cyanoborohydride, or sodium acetate, boron hydride, the difference is not so large, as long as you form the imine the next step is simply to restore.
6.The carbonyl group reacts with the amine imine (Schiff base), and then reduced to the amine with sodium borohydride or sodium cyanoborohydride. The reaction should be carried out under weakly acidic conditions, because on the one hand weakly acidic conditions of the carbonyl protonated enhances the electrophilicity of promoting the reaction, while avoiding excessive protonated amine nucleophilic cause decline occurred. Better than with sodium borohydride, sodium cyano boron hydride, a cyano group as electron-withdrawing inductive effect of hydrogen bonding weakens the activity of the boron, so cyano sodium borohydride reduction of Schiff base can be selectively without reducing aldehydes, ketones carbonyl group, thus avoiding side reactions. With NaBH (OAc) 3 as reducing agent used ClCH2CH2Cl as solvent and the reaction time can be shortened significantly increase yield.
Storage conditions: In case of oxygen deteriorate when wet, sealed.
Packing: 25KG / barrel
Product name: Sodium triacetoxyborohydride
Synonyms:stab;SODIUM TRIACETOXYBOROHYDRIDE;SODIUM TRIACETOXYHYDROBORATE; sodiumtriacetoxyborohydride,calselect?stab;Sodium Triacetoxyborohyride;Sodium triacetoborohydride;Sodium triacetohydroborate, Triacetohydroborate sodium salt;Sodiumtriacetoxyborohydride,95%
CAS: 56553-60-7
Formula: C6H10BNaO6
Molecular weight: 211.94
Chemical properties: triacetoxy sodium boron hydride, a white crystalline powder; soluble in benzene and tetrahydrofuran, ketones and aldehydes used for the reductive amination reaction of carbonyl compounds and amines reductive amination / lactamization, and aryl aldehyde reductive amination.
Soluble in benzene and tetrahydrofuran. Is milder selective borohydride reagents. Reacts with water, dissolved in THF, acetonitrile and 1,2-dichloroethane.
Melting point: 116-120 °C (dec.)(lit.)
Storage condition:Flammables + water-Freezer (-20°C)e area
Water solubility:reacts
Sensitivity:Moisture Sensitive
Merck :8695
BRN :4047608
Usage:
1.The new catalyst for the reductive amination with excellent universality and selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification, the catalyst itself and the byproducts are non-toxic, no environmental pollution, become reductive amination catalyst of choice.
2.Ketones and aldehydes used for the reduction reaction of an amine, carbonyl compounds and amines reductive amination / amidation inside, and aryl aldehyde reductive amination.
3.Triacetoxy sodium borohydride is a new catalyst for the reductive amination developed in recent years, because of its universality and excellent selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification the catalyst itself and the byproducts are non-toxic, no environmental pollution, as a catalyst for reductive amination preferred.
4.Catalytic hydrogenation in the metal catalyst of platinum, palladium, nickel or the like, although the method is more economical and has applied to the advantage of mass production, but got more than a mixture, and lower yields, as well as limitations on the structure carbon-carbon multiple bond can be reduced and there are functional groups present in the compounds, such as those containing nitro, cyano, catalytic hydrogenation method can not be used, and the catalytic performance is often inhibited by divalent sulfides.
5.Reductive amination refers to the conversion of carbonyl compounds to amines important organic reactions. Imine formed first, then reduced with sodium borohydride; either sodium borohydride, or triacetoxy sodium borohydride, sodium cyanoborohydride, or sodium acetate, boron hydride, the difference is not so large, as long as you form the imine the next step is simply to restore.
6.The carbonyl group reacts with the amine imine (Schiff base), and then reduced to the amine with sodium borohydride or sodium cyanoborohydride. The reaction should be carried out under weakly acidic conditions, because on the one hand weakly acidic conditions of the carbonyl protonated enhances the electrophilicity of promoting the reaction, while avoiding excessive protonated amine nucleophilic cause decline occurred. Better than with sodium borohydride, sodium cyano boron hydride, a cyano group as electron-withdrawing inductive effect of hydrogen bonding weakens the activity of the boron, so cyano sodium borohydride reduction of Schiff base can be selectively without reducing aldehydes, ketones carbonyl group, thus avoiding side reactions. With NaBH (OAc) 3 as reducing agent used ClCH2CH2Cl as solvent and the reaction time can be shortened significantly increase yield.
Storage conditions: In case of oxygen deteriorate when wet, sealed.
Packing: 25KG / barrel
World. Chem. Co., Ltd.
P.R.China
Product name: Sodium triacetoxyborohydride
Synonyms:stab;SODIUM TRIACETOXYBOROHYDRIDE;SODIUM TRIACETOXYHYDROBORATE; sodiumtriacetoxyborohydride,calselect?stab;Sodium Triacetoxyborohyride;Sodium triacetoborohydride;Sodium triacetohydroborate, Triacetohydroborate sodium salt;Sodiumtriacetoxyborohydride,95%
CAS: 56553-60-7
Formula: C6H10BNaO6
Molecular weight: 211.94
Chemical properties: triacetoxy sodium boron hydride, a white crystalline powder; soluble in benzene and tetrahydrofuran, ketones and aldehydes used for the reductive amination reaction of carbonyl compounds and amines reductive amination / lactamization, and aryl aldehyde reductive amination.
Soluble in benzene and tetrahydrofuran. Is milder selective borohydride reagents. Reacts with water, dissolved in THF, acetonitrile and 1,2-dichloroethane.
Melting point: 116-120 °C (dec.)(lit.)
Storage condition:Flammables + water-Freezer (-20°C)e area
Water solubility:reacts
Sensitivity:Moisture Sensitive
Merck :8695
BRN :4047608
Usage:
1.The new catalyst for the reductive amination with excellent universality and selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification, the catalyst itself and the byproducts are non-toxic, no environmental pollution, become reductive amination catalyst of choice.
2.Ketones and aldehydes used for the reduction reaction of an amine, carbonyl compounds and amines reductive amination / amidation inside, and aryl aldehyde reductive amination.
3.Triacetoxy sodium borohydride is a new catalyst for the reductive amination developed in recent years, because of its universality and excellent selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification the catalyst itself and the byproducts are non-toxic, no environmental pollution, as a catalyst for reductive amination preferred.
4.Catalytic hydrogenation in the metal catalyst of platinum, palladium, nickel or the like, although the method is more economical and has applied to the advantage of mass production, but got more than a mixture, and lower yields, as well as limitations on the structure carbon-carbon multiple bond can be reduced and there are functional groups present in the compounds, such as those containing nitro, cyano, catalytic hydrogenation method can not be used, and the catalytic performance is often inhibited by divalent sulfides.
5.Reductive amination refers to the conversion of carbonyl compounds to amines important organic reactions. Imine formed first, then reduced with sodium borohydride; either sodium borohydride, or triacetoxy sodium borohydride, sodium cyanoborohydride, or sodium acetate, boron hydride, the difference is not so large, as long as you form the imine the next step is simply to restore.
6.The carbonyl group reacts with the amine imine (Schiff base), and then reduced to the amine with sodium borohydride or sodium cyanoborohydride. The reaction should be carried out under weakly acidic conditions, because on the one hand weakly acidic conditions of the carbonyl protonated enhances the electrophilicity of promoting the reaction, while avoiding excessive protonated amine nucleophilic cause decline occurred. Better than with sodium borohydride, sodium cyano boron hydride, a cyano group as electron-withdrawing inductive effect of hydrogen bonding weakens the activity of the boron, so cyano sodium borohydride reduction of Schiff base can be selectively without reducing aldehydes, ketones carbonyl group, thus avoiding side reactions. With NaBH (OAc) 3 as reducing agent used ClCH2CH2Cl as solvent and the reaction time can be shortened significantly increase yield.
Storage conditions: In case of oxygen deteriorate when wet, sealed.
Packing: 25KG / barrel
Boulder Scientific Company
USA
Product name: Sodium triacetoxyborohydride
Synonyms:stab;SODIUM TRIACETOXYBOROHYDRIDE;SODIUM TRIACETOXYHYDROBORATE; sodiumtriacetoxyborohydride,calselect?stab;Sodium Triacetoxyborohyride;Sodium triacetoborohydride;Sodium triacetohydroborate, Triacetohydroborate sodium salt;Sodiumtriacetoxyborohydride,95%
CAS: 56553-60-7
Formula: C6H10BNaO6
Molecular weight: 211.94
Chemical properties: triacetoxy sodium boron hydride, a white crystalline powder; soluble in benzene and tetrahydrofuran, ketones and aldehydes used for the reductive amination reaction of carbonyl compounds and amines reductive amination / lactamization, and aryl aldehyde reductive amination.
Soluble in benzene and tetrahydrofuran. Is milder selective borohydride reagents. Reacts with water, dissolved in THF, acetonitrile and 1,2-dichloroethane.
Melting point: 116-120 °C (dec.)(lit.)
Storage condition:Flammables + water-Freezer (-20°C)e area
Water solubility:reacts
Sensitivity:Moisture Sensitive
Merck :8695
BRN :4047608
Usage:
1.The new catalyst for the reductive amination with excellent universality and selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification, the catalyst itself and the byproducts are non-toxic, no environmental pollution, become reductive amination catalyst of choice.
2.Ketones and aldehydes used for the reduction reaction of an amine, carbonyl compounds and amines reductive amination / amidation inside, and aryl aldehyde reductive amination.
3.Triacetoxy sodium borohydride is a new catalyst for the reductive amination developed in recent years, because of its universality and excellent selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification the catalyst itself and the byproducts are non-toxic, no environmental pollution, as a catalyst for reductive amination preferred.
4.Catalytic hydrogenation in the metal catalyst of platinum, palladium, nickel or the like, although the method is more economical and has applied to the advantage of mass production, but got more than a mixture, and lower yields, as well as limitations on the structure carbon-carbon multiple bond can be reduced and there are functional groups present in the compounds, such as those containing nitro, cyano, catalytic hydrogenation method can not be used, and the catalytic performance is often inhibited by divalent sulfides.
5.Reductive amination refers to the conversion of carbonyl compounds to amines important organic reactions. Imine formed first, then reduced with sodium borohydride; either sodium borohydride, or triacetoxy sodium borohydride, sodium cyanoborohydride, or sodium acetate, boron hydride, the difference is not so large, as long as you form the imine the next step is simply to restore.
6.The carbonyl group reacts with the amine imine (Schiff base), and then reduced to the amine with sodium borohydride or sodium cyanoborohydride. The reaction should be carried out under weakly acidic conditions, because on the one hand weakly acidic conditions of the carbonyl protonated enhances the electrophilicity of promoting the reaction, while avoiding excessive protonated amine nucleophilic cause decline occurred. Better than with sodium borohydride, sodium cyano boron hydride, a cyano group as electron-withdrawing inductive effect of hydrogen bonding weakens the activity of the boron, so cyano sodium borohydride reduction of Schiff base can be selectively without reducing aldehydes, ketones carbonyl group, thus avoiding side reactions. With NaBH (OAc) 3 as reducing agent used ClCH2CH2Cl as solvent and the reaction time can be shortened significantly increase yield.
Storage conditions: In case of oxygen deteriorate when wet, sealed.
Packing: 25KG / barrel
FAR Chemical
USA
Product name: Sodium triacetoxyborohydride
Synonyms:stab;SODIUM TRIACETOXYBOROHYDRIDE;SODIUM TRIACETOXYHYDROBORATE; sodiumtriacetoxyborohydride,calselect?stab;Sodium Triacetoxyborohyride;Sodium triacetoborohydride;Sodium triacetohydroborate, Triacetohydroborate sodium salt;Sodiumtriacetoxyborohydride,95%
CAS: 56553-60-7
Formula: C6H10BNaO6
Molecular weight: 211.94
Chemical properties: triacetoxy sodium boron hydride, a white crystalline powder; soluble in benzene and tetrahydrofuran, ketones and aldehydes used for the reductive amination reaction of carbonyl compounds and amines reductive amination / lactamization, and aryl aldehyde reductive amination.
Soluble in benzene and tetrahydrofuran. Is milder selective borohydride reagents. Reacts with water, dissolved in THF, acetonitrile and 1,2-dichloroethane.
Melting point: 116-120 °C (dec.)(lit.)
Storage condition:Flammables + water-Freezer (-20°C)e area
Water solubility:reacts
Sensitivity:Moisture Sensitive
Merck :8695
BRN :4047608
Usage:
1.The new catalyst for the reductive amination with excellent universality and selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification, the catalyst itself and the byproducts are non-toxic, no environmental pollution, become reductive amination catalyst of choice.
2.Ketones and aldehydes used for the reduction reaction of an amine, carbonyl compounds and amines reductive amination / amidation inside, and aryl aldehyde reductive amination.
3.Triacetoxy sodium borohydride is a new catalyst for the reductive amination developed in recent years, because of its universality and excellent selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification the catalyst itself and the byproducts are non-toxic, no environmental pollution, as a catalyst for reductive amination preferred.
4.Catalytic hydrogenation in the metal catalyst of platinum, palladium, nickel or the like, although the method is more economical and has applied to the advantage of mass production, but got more than a mixture, and lower yields, as well as limitations on the structure carbon-carbon multiple bond can be reduced and there are functional groups present in the compounds, such as those containing nitro, cyano, catalytic hydrogenation method can not be used, and the catalytic performance is often inhibited by divalent sulfides.
5.Reductive amination refers to the conversion of carbonyl compounds to amines important organic reactions. Imine formed first, then reduced with sodium borohydride; either sodium borohydride, or triacetoxy sodium borohydride, sodium cyanoborohydride, or sodium acetate, boron hydride, the difference is not so large, as long as you form the imine the next step is simply to restore.
6.The carbonyl group reacts with the amine imine (Schiff base), and then reduced to the amine with sodium borohydride or sodium cyanoborohydride. The reaction should be carried out under weakly acidic conditions, because on the one hand weakly acidic conditions of the carbonyl protonated enhances the electrophilicity of promoting the reaction, while avoiding excessive protonated amine nucleophilic cause decline occurred. Better than with sodium borohydride, sodium cyano boron hydride, a cyano group as electron-withdrawing inductive effect of hydrogen bonding weakens the activity of the boron, so cyano sodium borohydride reduction of Schiff base can be selectively without reducing aldehydes, ketones carbonyl group, thus avoiding side reactions. With NaBH (OAc) 3 as reducing agent used ClCH2CH2Cl as solvent and the reaction time can be shortened significantly increase yield.
Storage conditions: In case of oxygen deteriorate when wet, sealed.
Packing: 25KG / barrel
Merck Schuchardt OHG
Germany
Product name: Sodium triacetoxyborohydride
Synonyms:stab;SODIUM TRIACETOXYBOROHYDRIDE;SODIUM TRIACETOXYHYDROBORATE; sodiumtriacetoxyborohydride,calselect?stab;Sodium Triacetoxyborohyride;Sodium triacetoborohydride;Sodium triacetohydroborate, Triacetohydroborate sodium salt;Sodiumtriacetoxyborohydride,95%
CAS: 56553-60-7
Formula: C6H10BNaO6
Molecular weight: 211.94
Chemical properties: triacetoxy sodium boron hydride, a white crystalline powder; soluble in benzene and tetrahydrofuran, ketones and aldehydes used for the reductive amination reaction of carbonyl compounds and amines reductive amination / lactamization, and aryl aldehyde reductive amination.
Soluble in benzene and tetrahydrofuran. Is milder selective borohydride reagents. Reacts with water, dissolved in THF, acetonitrile and 1,2-dichloroethane.
Melting point: 116-120 °C (dec.)(lit.)
Storage condition:Flammables + water-Freezer (-20°C)e area
Water solubility:reacts
Sensitivity:Moisture Sensitive
Merck :8695
BRN :4047608
Usage:
1.The new catalyst for the reductive amination with excellent universality and selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification, the catalyst itself and the byproducts are non-toxic, no environmental pollution, become reductive amination catalyst of choice.
2.Ketones and aldehydes used for the reduction reaction of an amine, carbonyl compounds and amines reductive amination / amidation inside, and aryl aldehyde reductive amination.
3.Triacetoxy sodium borohydride is a new catalyst for the reductive amination developed in recent years, because of its universality and excellent selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification the catalyst itself and the byproducts are non-toxic, no environmental pollution, as a catalyst for reductive amination preferred.
4.Catalytic hydrogenation in the metal catalyst of platinum, palladium, nickel or the like, although the method is more economical and has applied to the advantage of mass production, but got more than a mixture, and lower yields, as well as limitations on the structure carbon-carbon multiple bond can be reduced and there are functional groups present in the compounds, such as those containing nitro, cyano, catalytic hydrogenation method can not be used, and the catalytic performance is often inhibited by divalent sulfides.
5.Reductive amination refers to the conversion of carbonyl compounds to amines important organic reactions. Imine formed first, then reduced with sodium borohydride; either sodium borohydride, or triacetoxy sodium borohydride, sodium cyanoborohydride, or sodium acetate, boron hydride, the difference is not so large, as long as you form the imine the next step is simply to restore.
6.The carbonyl group reacts with the amine imine (Schiff base), and then reduced to the amine with sodium borohydride or sodium cyanoborohydride. The reaction should be carried out under weakly acidic conditions, because on the one hand weakly acidic conditions of the carbonyl protonated enhances the electrophilicity of promoting the reaction, while avoiding excessive protonated amine nucleophilic cause decline occurred. Better than with sodium borohydride, sodium cyano boron hydride, a cyano group as electron-withdrawing inductive effect of hydrogen bonding weakens the activity of the boron, so cyano sodium borohydride reduction of Schiff base can be selectively without reducing aldehydes, ketones carbonyl group, thus avoiding side reactions. With NaBH (OAc) 3 as reducing agent used ClCH2CH2Cl as solvent and the reaction time can be shortened significantly increase yield.
Storage conditions: In case of oxygen deteriorate when wet, sealed.
Packing: 25KG / barrel
CM Fine Chemicals
Switzerland
Product name: Sodium triacetoxyborohydride
Synonyms:stab;SODIUM TRIACETOXYBOROHYDRIDE;SODIUM TRIACETOXYHYDROBORATE; sodiumtriacetoxyborohydride,calselect?stab;Sodium Triacetoxyborohyride;Sodium triacetoborohydride;Sodium triacetohydroborate, Triacetohydroborate sodium salt;Sodiumtriacetoxyborohydride,95%
CAS: 56553-60-7
Formula: C6H10BNaO6
Molecular weight: 211.94
Chemical properties: triacetoxy sodium boron hydride, a white crystalline powder; soluble in benzene and tetrahydrofuran, ketones and aldehydes used for the reductive amination reaction of carbonyl compounds and amines reductive amination / lactamization, and aryl aldehyde reductive amination.
Soluble in benzene and tetrahydrofuran. Is milder selective borohydride reagents. Reacts with water, dissolved in THF, acetonitrile and 1,2-dichloroethane.
Melting point: 116-120 °C (dec.)(lit.)
Storage condition:Flammables + water-Freezer (-20°C)e area
Water solubility:reacts
Sensitivity:Moisture Sensitive
Merck :8695
BRN :4047608
Usage:
1.The new catalyst for the reductive amination with excellent universality and selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification, the catalyst itself and the byproducts are non-toxic, no environmental pollution, become reductive amination catalyst of choice.
2.Ketones and aldehydes used for the reduction reaction of an amine, carbonyl compounds and amines reductive amination / amidation inside, and aryl aldehyde reductive amination.
3.Triacetoxy sodium borohydride is a new catalyst for the reductive amination developed in recent years, because of its universality and excellent selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification the catalyst itself and the byproducts are non-toxic, no environmental pollution, as a catalyst for reductive amination preferred.
4.Catalytic hydrogenation in the metal catalyst of platinum, palladium, nickel or the like, although the method is more economical and has applied to the advantage of mass production, but got more than a mixture, and lower yields, as well as limitations on the structure carbon-carbon multiple bond can be reduced and there are functional groups present in the compounds, such as those containing nitro, cyano, catalytic hydrogenation method can not be used, and the catalytic performance is often inhibited by divalent sulfides.
5.Reductive amination refers to the conversion of carbonyl compounds to amines important organic reactions. Imine formed first, then reduced with sodium borohydride; either sodium borohydride, or triacetoxy sodium borohydride, sodium cyanoborohydride, or sodium acetate, boron hydride, the difference is not so large, as long as you form the imine the next step is simply to restore.
6.The carbonyl group reacts with the amine imine (Schiff base), and then reduced to the amine with sodium borohydride or sodium cyanoborohydride. The reaction should be carried out under weakly acidic conditions, because on the one hand weakly acidic conditions of the carbonyl protonated enhances the electrophilicity of promoting the reaction, while avoiding excessive protonated amine nucleophilic cause decline occurred. Better than with sodium borohydride, sodium cyano boron hydride, a cyano group as electron-withdrawing inductive effect of hydrogen bonding weakens the activity of the boron, so cyano sodium borohydride reduction of Schiff base can be selectively without reducing aldehydes, ketones carbonyl group, thus avoiding side reactions. With NaBH (OAc) 3 as reducing agent used ClCH2CH2Cl as solvent and the reaction time can be shortened significantly increase yield.
Storage conditions: In case of oxygen deteriorate when wet, sealed.
Packing: 25KG / barrel
Santa Cruz Biotechnology, Inc.
USA
Product name: Sodium triacetoxyborohydride
Synonyms:stab;SODIUM TRIACETOXYBOROHYDRIDE;SODIUM TRIACETOXYHYDROBORATE; sodiumtriacetoxyborohydride,calselect?stab;Sodium Triacetoxyborohyride;Sodium triacetoborohydride;Sodium triacetohydroborate, Triacetohydroborate sodium salt;Sodiumtriacetoxyborohydride,95%
CAS: 56553-60-7
Formula: C6H10BNaO6
Molecular weight: 211.94
Chemical properties: triacetoxy sodium boron hydride, a white crystalline powder; soluble in benzene and tetrahydrofuran, ketones and aldehydes used for the reductive amination reaction of carbonyl compounds and amines reductive amination / lactamization, and aryl aldehyde reductive amination.
Soluble in benzene and tetrahydrofuran. Is milder selective borohydride reagents. Reacts with water, dissolved in THF, acetonitrile and 1,2-dichloroethane.
Melting point: 116-120 °C (dec.)(lit.)
Storage condition:Flammables + water-Freezer (-20°C)e area
Water solubility:reacts
Sensitivity:Moisture Sensitive
Merck :8695
BRN :4047608
Usage:
1.The new catalyst for the reductive amination with excellent universality and selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification, the catalyst itself and the byproducts are non-toxic, no environmental pollution, become reductive amination catalyst of choice.
2.Ketones and aldehydes used for the reduction reaction of an amine, carbonyl compounds and amines reductive amination / amidation inside, and aryl aldehyde reductive amination.
3.Triacetoxy sodium borohydride is a new catalyst for the reductive amination developed in recent years, because of its universality and excellent selectivity, mild reaction conditions, good catalytic reduction performance, and easy separation and purification the catalyst itself and the byproducts are non-toxic, no environmental pollution, as a catalyst for reductive amination preferred.
4.Catalytic hydrogenation in the metal catalyst of platinum, palladium, nickel or the like, although the method is more economical and has applied to the advantage of mass production, but got more than a mixture, and lower yields, as well as limitations on the structure carbon-carbon multiple bond can be reduced and there are functional groups present in the compounds, such as those containing nitro, cyano, catalytic hydrogenation method can not be used, and the catalytic performance is often inhibited by divalent sulfides.
5.Reductive amination refers to the conversion of carbonyl compounds to amines important organic reactions. Imine formed first, then reduced with sodium borohydride; either sodium borohydride, or triacetoxy sodium borohydride, sodium cyanoborohydride, or sodium acetate, boron hydride, the difference is not so large, as long as you form the imine the next step is simply to restore.
6.The carbonyl group reacts with the amine imine (Schiff base), and then reduced to the amine with sodium borohydride or sodium cyanoborohydride. The reaction should be carried out under weakly acidic conditions, because on the one hand weakly acidic conditions of the carbonyl protonated enhances the electrophilicity of promoting the reaction, while avoiding excessive protonated amine nucleophilic cause decline occurred. Better than with sodium borohydride, sodium cyano boron hydride, a cyano group as electron-withdrawing inductive effect of hydrogen bonding weakens the activity of the boron, so cyano sodium borohydride reduction of Schiff base can be selectively without reducing aldehydes, ketones carbonyl group, thus avoiding side reactions. With NaBH (OAc) 3 as reducing agent used ClCH2CH2Cl as solvent and the reaction time can be shortened significantly increase yield.
Storage conditions: In case of oxygen deteriorate when wet, sealed.
Packing: 25KG / barrel
|


 details
details details
details details
details



_Co_Ltd_1_575.jpg)




