|
Suppliers for CAS
123171-59-5
|
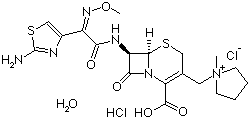
Properties | | CAS |
123171-59-5 | | Formula |
C19H24N6O5S2 . 2HCl |
|
|
18 Registered suppliers
Dayang Chem (Hangzhou) Co.,Ltd.
P.R.China
Hangzhou Meite Industry Co., Ltd (Hangzhou Meite Chemical Co., Ltd)
P.R.China
Leap Chem Co., Ltd
P.R.China
Molecular Formula: C19H24N6O5S2.2ClH.H2O Molecular Weight: 571.5 Hazard Symbols: Xi WGKGermany: 3 HS Code: 29419000
More details are to be found here
Sigma-Aldrich International GmbH
Switzerland
Molecular Formula: C19H24N6O5S2.2ClH.H2O Molecular Weight: 571.5 Hazard Symbols: Xi WGKGermany: 3 HS Code: 29419000
More details are to be found here
X´ian Taicheng Chem Co., Ltd
P.R.China
Molecular Formula: C19H24N6O5S2.2ClH.H2O Molecular Weight: 571.5 Hazard Symbols: Xi WGKGermany: 3 HS Code: 29419000
More details are to be found here
Hangzhou Zhongqi Chem Co., Ltd
P.R.China
Molecular Formula: C19H24N6O5S2.2ClH.H2O Molecular Weight: 571.5 Hazard Symbols: Xi WGKGermany: 3 HS Code: 29419000
More details are to be found here
Nantong Xindao Biotech Ltd
P.R.China
Molecular Formula: C19H24N6O5S2.2ClH.H2O Molecular Weight: 571.5 Hazard Symbols: Xi WGKGermany: 3 HS Code: 29419000
More details are to be found here
Hangzhou Lingrui Chemical Co., Ltd.
P.R.China
Molecular Formula: C19H24N6O5S2.2ClH.H2O Molecular Weight: 571.5 Hazard Symbols: Xi WGKGermany: 3 HS Code: 29419000
More details are to be found here
Skyrun Industrial Co., Ltd.
P.R.China
Molecular Formula: C19H24N6O5S2.2ClH.H2O Molecular Weight: 571.5 Hazard Symbols: Xi WGKGermany: 3 HS Code: 29419000
More details are to be found here
BOC Sciences
USA
Description : Cefepime is an extended-spectrum parenteral cephalosporin antibiotic active in vitro against a broad spectrum of gram-positive and gram-negative aerobic bacteria. Cefepime dosing was 1-4 g/day (0.5-2.0 g twice daily) for adults; ceftazidime dosing was 1-6 g/day (0.5 g every 12 hours to 2.0 g every 8 hours). A limited number of cefepime-treated patients received 2 g every 8 hours. The median length of dosing for both cefepime and ceftazidime was 7 days. Cefepime has a decreased propensity to induce beta-lactamases compared with other beta-lactam antibiotics. Cefepime has a pharmacokinetic disposition similar to that of other renally eliminated cephalosporins, with a half-life of approximately 2 hours. Cefepime has demonstrated clinical efficacy against a variety of infections, including urinary tract infections, pneumonia, and skin structure infections. Cefepime is generally well tolerated. - Molecular Weight :571.50
- Melting Point :150°C(dec.)
- Purity :> 98%
Molecular Formula : C19H28Cl2N6O6S2 Canonical SMILES : C[N+]1(CCCC1)CC2=C(N3C(C(C3=O)NC(=O)C(=NOC)C4=CSC(=N4)N)SC2)C(=O)O.O.Cl.[Cl-]
InChI :
InChI=1S/C19H24N6O5S2.2ClH.H2O/c1-25(5-3-4-6-25)7-10-8-31-17-13(16(27)24(17)14(10)18(28)29)22-15(26)12(23-30-2)11-9-32-19(20)21-11;;;/h9,13,17H,3-8H2,1-2H3,(H3-,20,21,22,26,28,29);2*1H;1H2/b23-12-;;;/t13-,17-;;;/m1./s1
InChIKey :
LRAJHPGSGBRUJN-OMIVUECESA-N
Solubility :
Soluble in DMSO
Appearance :
White Solid
Storage : Store at -20°C
Synonyms :
Maxipime
More details are to be found here
Shandong SanYoung Industry Co., Ltd
P.R.China
Description : Cefepime is an extended-spectrum parenteral cephalosporin antibiotic active in vitro against a broad spectrum of gram-positive and gram-negative aerobic bacteria. Cefepime dosing was 1-4 g/day (0.5-2.0 g twice daily) for adults; ceftazidime dosing was 1-6 g/day (0.5 g every 12 hours to 2.0 g every 8 hours). A limited number of cefepime-treated patients received 2 g every 8 hours. The median length of dosing for both cefepime and ceftazidime was 7 days. Cefepime has a decreased propensity to induce beta-lactamases compared with other beta-lactam antibiotics. Cefepime has a pharmacokinetic disposition similar to that of other renally eliminated cephalosporins, with a half-life of approximately 2 hours. Cefepime has demonstrated clinical efficacy against a variety of infections, including urinary tract infections, pneumonia, and skin structure infections. Cefepime is generally well tolerated. - Molecular Weight :571.50
- Melting Point :150°C(dec.)
- Purity :> 98%
Molecular Formula : C19H28Cl2N6O6S2 Canonical SMILES : C[N+]1(CCCC1)CC2=C(N3C(C(C3=O)NC(=O)C(=NOC)C4=CSC(=N4)N)SC2)C(=O)O.O.Cl.[Cl-]
InChI :
InChI=1S/C19H24N6O5S2.2ClH.H2O/c1-25(5-3-4-6-25)7-10-8-31-17-13(16(27)24(17)14(10)18(28)29)22-15(26)12(23-30-2)11-9-32-19(20)21-11;;;/h9,13,17H,3-8H2,1-2H3,(H3-,20,21,22,26,28,29);2*1H;1H2/b23-12-;;;/t13-,17-;;;/m1./s1
InChIKey :
LRAJHPGSGBRUJN-OMIVUECESA-N
Solubility :
Soluble in DMSO
Appearance :
White Solid
Storage : Store at -20°C
Synonyms :
Maxipime
More details are to be found here
Carbone Scientific Co., Ltd.
U.K.
Description : Cefepime is an extended-spectrum parenteral cephalosporin antibiotic active in vitro against a broad spectrum of gram-positive and gram-negative aerobic bacteria. Cefepime dosing was 1-4 g/day (0.5-2.0 g twice daily) for adults; ceftazidime dosing was 1-6 g/day (0.5 g every 12 hours to 2.0 g every 8 hours). A limited number of cefepime-treated patients received 2 g every 8 hours. The median length of dosing for both cefepime and ceftazidime was 7 days. Cefepime has a decreased propensity to induce beta-lactamases compared with other beta-lactam antibiotics. Cefepime has a pharmacokinetic disposition similar to that of other renally eliminated cephalosporins, with a half-life of approximately 2 hours. Cefepime has demonstrated clinical efficacy against a variety of infections, including urinary tract infections, pneumonia, and skin structure infections. Cefepime is generally well tolerated. - Molecular Weight :571.50
- Melting Point :150°C(dec.)
- Purity :> 98%
Molecular Formula : C19H28Cl2N6O6S2 Canonical SMILES : C[N+]1(CCCC1)CC2=C(N3C(C(C3=O)NC(=O)C(=NOC)C4=CSC(=N4)N)SC2)C(=O)O.O.Cl.[Cl-]
InChI :
InChI=1S/C19H24N6O5S2.2ClH.H2O/c1-25(5-3-4-6-25)7-10-8-31-17-13(16(27)24(17)14(10)18(28)29)22-15(26)12(23-30-2)11-9-32-19(20)21-11;;;/h9,13,17H,3-8H2,1-2H3,(H3-,20,21,22,26,28,29);2*1H;1H2/b23-12-;;;/t13-,17-;;;/m1./s1
InChIKey :
LRAJHPGSGBRUJN-OMIVUECESA-N
Solubility :
Soluble in DMSO
Appearance :
White Solid
Storage : Store at -20°C
Synonyms :
Maxipime
More details are to be found here
Xiamen Equation Chemical Co.,Ltd
P.R.China
Description : Cefepime is an extended-spectrum parenteral cephalosporin antibiotic active in vitro against a broad spectrum of gram-positive and gram-negative aerobic bacteria. Cefepime dosing was 1-4 g/day (0.5-2.0 g twice daily) for adults; ceftazidime dosing was 1-6 g/day (0.5 g every 12 hours to 2.0 g every 8 hours). A limited number of cefepime-treated patients received 2 g every 8 hours. The median length of dosing for both cefepime and ceftazidime was 7 days. Cefepime has a decreased propensity to induce beta-lactamases compared with other beta-lactam antibiotics. Cefepime has a pharmacokinetic disposition similar to that of other renally eliminated cephalosporins, with a half-life of approximately 2 hours. Cefepime has demonstrated clinical efficacy against a variety of infections, including urinary tract infections, pneumonia, and skin structure infections. Cefepime is generally well tolerated. - Molecular Weight :571.50
- Melting Point :150°C(dec.)
- Purity :> 98%
Molecular Formula : C19H28Cl2N6O6S2 Canonical SMILES : C[N+]1(CCCC1)CC2=C(N3C(C(C3=O)NC(=O)C(=NOC)C4=CSC(=N4)N)SC2)C(=O)O.O.Cl.[Cl-]
InChI :
InChI=1S/C19H24N6O5S2.2ClH.H2O/c1-25(5-3-4-6-25)7-10-8-31-17-13(16(27)24(17)14(10)18(28)29)22-15(26)12(23-30-2)11-9-32-19(20)21-11;;;/h9,13,17H,3-8H2,1-2H3,(H3-,20,21,22,26,28,29);2*1H;1H2/b23-12-;;;/t13-,17-;;;/m1./s1
InChIKey :
LRAJHPGSGBRUJN-OMIVUECESA-N
Solubility :
Soluble in DMSO
Appearance :
White Solid
Storage : Store at -20°C
Synonyms :
Maxipime
More details are to be found here
AK Scientific, Inc.
USA
Description : Cefepime is an extended-spectrum parenteral cephalosporin antibiotic active in vitro against a broad spectrum of gram-positive and gram-negative aerobic bacteria. Cefepime dosing was 1-4 g/day (0.5-2.0 g twice daily) for adults; ceftazidime dosing was 1-6 g/day (0.5 g every 12 hours to 2.0 g every 8 hours). A limited number of cefepime-treated patients received 2 g every 8 hours. The median length of dosing for both cefepime and ceftazidime was 7 days. Cefepime has a decreased propensity to induce beta-lactamases compared with other beta-lactam antibiotics. Cefepime has a pharmacokinetic disposition similar to that of other renally eliminated cephalosporins, with a half-life of approximately 2 hours. Cefepime has demonstrated clinical efficacy against a variety of infections, including urinary tract infections, pneumonia, and skin structure infections. Cefepime is generally well tolerated. - Molecular Weight :571.50
- Melting Point :150°C(dec.)
- Purity :> 98%
Molecular Formula : C19H28Cl2N6O6S2 Canonical SMILES : C[N+]1(CCCC1)CC2=C(N3C(C(C3=O)NC(=O)C(=NOC)C4=CSC(=N4)N)SC2)C(=O)O.O.Cl.[Cl-]
InChI :
InChI=1S/C19H24N6O5S2.2ClH.H2O/c1-25(5-3-4-6-25)7-10-8-31-17-13(16(27)24(17)14(10)18(28)29)22-15(26)12(23-30-2)11-9-32-19(20)21-11;;;/h9,13,17H,3-8H2,1-2H3,(H3-,20,21,22,26,28,29);2*1H;1H2/b23-12-;;;/t13-,17-;;;/m1./s1
InChIKey :
LRAJHPGSGBRUJN-OMIVUECESA-N
Solubility :
Soluble in DMSO
Appearance :
White Solid
Storage : Store at -20°C
Synonyms :
Maxipime
More details are to be found here
Chemos GmbH & Co. KG
Germany
Description : Cefepime is an extended-spectrum parenteral cephalosporin antibiotic active in vitro against a broad spectrum of gram-positive and gram-negative aerobic bacteria. Cefepime dosing was 1-4 g/day (0.5-2.0 g twice daily) for adults; ceftazidime dosing was 1-6 g/day (0.5 g every 12 hours to 2.0 g every 8 hours). A limited number of cefepime-treated patients received 2 g every 8 hours. The median length of dosing for both cefepime and ceftazidime was 7 days. Cefepime has a decreased propensity to induce beta-lactamases compared with other beta-lactam antibiotics. Cefepime has a pharmacokinetic disposition similar to that of other renally eliminated cephalosporins, with a half-life of approximately 2 hours. Cefepime has demonstrated clinical efficacy against a variety of infections, including urinary tract infections, pneumonia, and skin structure infections. Cefepime is generally well tolerated. - Molecular Weight :571.50
- Melting Point :150°C(dec.)
- Purity :> 98%
Molecular Formula : C19H28Cl2N6O6S2 Canonical SMILES : C[N+]1(CCCC1)CC2=C(N3C(C(C3=O)NC(=O)C(=NOC)C4=CSC(=N4)N)SC2)C(=O)O.O.Cl.[Cl-]
InChI :
InChI=1S/C19H24N6O5S2.2ClH.H2O/c1-25(5-3-4-6-25)7-10-8-31-17-13(16(27)24(17)14(10)18(28)29)22-15(26)12(23-30-2)11-9-32-19(20)21-11;;;/h9,13,17H,3-8H2,1-2H3,(H3-,20,21,22,26,28,29);2*1H;1H2/b23-12-;;;/t13-,17-;;;/m1./s1
InChIKey :
LRAJHPGSGBRUJN-OMIVUECESA-N
Solubility :
Soluble in DMSO
Appearance :
White Solid
Storage : Store at -20°C
Synonyms :
Maxipime
More details are to be found here
BuGuCh & Partners
Germany
Description : Cefepime is an extended-spectrum parenteral cephalosporin antibiotic active in vitro against a broad spectrum of gram-positive and gram-negative aerobic bacteria. Cefepime dosing was 1-4 g/day (0.5-2.0 g twice daily) for adults; ceftazidime dosing was 1-6 g/day (0.5 g every 12 hours to 2.0 g every 8 hours). A limited number of cefepime-treated patients received 2 g every 8 hours. The median length of dosing for both cefepime and ceftazidime was 7 days. Cefepime has a decreased propensity to induce beta-lactamases compared with other beta-lactam antibiotics. Cefepime has a pharmacokinetic disposition similar to that of other renally eliminated cephalosporins, with a half-life of approximately 2 hours. Cefepime has demonstrated clinical efficacy against a variety of infections, including urinary tract infections, pneumonia, and skin structure infections. Cefepime is generally well tolerated. - Molecular Weight :571.50
- Melting Point :150°C(dec.)
- Purity :> 98%
Molecular Formula : C19H28Cl2N6O6S2 Canonical SMILES : C[N+]1(CCCC1)CC2=C(N3C(C(C3=O)NC(=O)C(=NOC)C4=CSC(=N4)N)SC2)C(=O)O.O.Cl.[Cl-]
InChI :
InChI=1S/C19H24N6O5S2.2ClH.H2O/c1-25(5-3-4-6-25)7-10-8-31-17-13(16(27)24(17)14(10)18(28)29)22-15(26)12(23-30-2)11-9-32-19(20)21-11;;;/h9,13,17H,3-8H2,1-2H3,(H3-,20,21,22,26,28,29);2*1H;1H2/b23-12-;;;/t13-,17-;;;/m1./s1
InChIKey :
LRAJHPGSGBRUJN-OMIVUECESA-N
Solubility :
Soluble in DMSO
Appearance :
White Solid
Storage : Store at -20°C
Synonyms :
Maxipime
More details are to be found here
CM Fine Chemicals
Switzerland
Description : Cefepime is an extended-spectrum parenteral cephalosporin antibiotic active in vitro against a broad spectrum of gram-positive and gram-negative aerobic bacteria. Cefepime dosing was 1-4 g/day (0.5-2.0 g twice daily) for adults; ceftazidime dosing was 1-6 g/day (0.5 g every 12 hours to 2.0 g every 8 hours). A limited number of cefepime-treated patients received 2 g every 8 hours. The median length of dosing for both cefepime and ceftazidime was 7 days. Cefepime has a decreased propensity to induce beta-lactamases compared with other beta-lactam antibiotics. Cefepime has a pharmacokinetic disposition similar to that of other renally eliminated cephalosporins, with a half-life of approximately 2 hours. Cefepime has demonstrated clinical efficacy against a variety of infections, including urinary tract infections, pneumonia, and skin structure infections. Cefepime is generally well tolerated. - Molecular Weight :571.50
- Melting Point :150°C(dec.)
- Purity :> 98%
Molecular Formula : C19H28Cl2N6O6S2 Canonical SMILES : C[N+]1(CCCC1)CC2=C(N3C(C(C3=O)NC(=O)C(=NOC)C4=CSC(=N4)N)SC2)C(=O)O.O.Cl.[Cl-]
InChI :
InChI=1S/C19H24N6O5S2.2ClH.H2O/c1-25(5-3-4-6-25)7-10-8-31-17-13(16(27)24(17)14(10)18(28)29)22-15(26)12(23-30-2)11-9-32-19(20)21-11;;;/h9,13,17H,3-8H2,1-2H3,(H3-,20,21,22,26,28,29);2*1H;1H2/b23-12-;;;/t13-,17-;;;/m1./s1
InChIKey :
LRAJHPGSGBRUJN-OMIVUECESA-N
Solubility :
Soluble in DMSO
Appearance :
White Solid
Storage : Store at -20°C
Synonyms :
Maxipime
More details are to be found here
Santa Cruz Biotechnology, Inc.
USA
Description : Cefepime is an extended-spectrum parenteral cephalosporin antibiotic active in vitro against a broad spectrum of gram-positive and gram-negative aerobic bacteria. Cefepime dosing was 1-4 g/day (0.5-2.0 g twice daily) for adults; ceftazidime dosing was 1-6 g/day (0.5 g every 12 hours to 2.0 g every 8 hours). A limited number of cefepime-treated patients received 2 g every 8 hours. The median length of dosing for both cefepime and ceftazidime was 7 days. Cefepime has a decreased propensity to induce beta-lactamases compared with other beta-lactam antibiotics. Cefepime has a pharmacokinetic disposition similar to that of other renally eliminated cephalosporins, with a half-life of approximately 2 hours. Cefepime has demonstrated clinical efficacy against a variety of infections, including urinary tract infections, pneumonia, and skin structure infections. Cefepime is generally well tolerated. - Molecular Weight :571.50
- Melting Point :150°C(dec.)
- Purity :> 98%
Molecular Formula : C19H28Cl2N6O6S2 Canonical SMILES : C[N+]1(CCCC1)CC2=C(N3C(C(C3=O)NC(=O)C(=NOC)C4=CSC(=N4)N)SC2)C(=O)O.O.Cl.[Cl-]
InChI :
InChI=1S/C19H24N6O5S2.2ClH.H2O/c1-25(5-3-4-6-25)7-10-8-31-17-13(16(27)24(17)14(10)18(28)29)22-15(26)12(23-30-2)11-9-32-19(20)21-11;;;/h9,13,17H,3-8H2,1-2H3,(H3-,20,21,22,26,28,29);2*1H;1H2/b23-12-;;;/t13-,17-;;;/m1./s1
InChIKey :
LRAJHPGSGBRUJN-OMIVUECESA-N
Solubility :
Soluble in DMSO
Appearance :
White Solid
Storage : Store at -20°C
Synonyms :
Maxipime
More details are to be found here
|


 details
details details
details_Co_Ltd_1_575.jpg)








