|
Suppliers for CAS
7446-08-4
|
Properties | | CAS |
7446-08-4 | | Formula |
SeO2 | | EINECS |
231-194-7 |
|
31 Registered suppliers
Simagchem Corporation
P.R.China
Gansu Jinchuan Liuzu New Material Application Science & Technology Co.
P.R.China
Purity: > /= 98%
Package: 25Kg/drum
MOQ: 25Kg
Delivery Time: 5 days (Transportation and Sailing Time is not included)
Supply ability: 600T/y
Aecochem Corp.
P.R.China
Purity: > /= 98%
Package: 25Kg/drum
MOQ: 25Kg
Delivery Time: 5 days (Transportation and Sailing Time is not included)
Supply ability: 600T/y
Dayang Chem (Hangzhou) Co.,Ltd.
P.R.China
Purity: > /= 98%
Package: 25Kg/drum
MOQ: 25Kg
Delivery Time: 5 days (Transportation and Sailing Time is not included)
Supply ability: 600T/y
Sancai Industry Co.,Ltd.
P.R.China
| Appearance | White crystal
| | Selenium Dioxide | ≥ 98.0 %
| | Hydrochloric acid insoluble | ≤ 0.02 %
| | Fe | ≤ 0.002 %
| | Loss on drying | ≤ 1.0 %
| | Selenate and sulphate | ≤ 0.1 %
| | Water dissolution test | Eligible
| | Chloride (Cl) | ≤ 0.005%
| | Heavy metal | ≤ 0.06%
| | Standard | Technical grade
| | Capacity | 100MT/month
| | Loading port | Shanghai
| | Use | Selenium dioxide can be used as manganese electrolysis additive, microelement additive for beverage, blackening agent for steal material.Selenium dioxide can be used to deposit zirconium, hafnium and selenium compounds
| | Package | 25kg/drum,or according to the customers’ demand
| | Storage | Stored in the dry and ventilated inside storeroom, prevent direct sunlight, slightly pile and put down |
Hangzhou Keying Chem Co., Ltd.
P.R.China
| Appearance | White crystal
| | Selenium Dioxide | ≥ 98.0 %
| | Hydrochloric acid insoluble | ≤ 0.02 %
| | Fe | ≤ 0.002 %
| | Loss on drying | ≤ 1.0 %
| | Selenate and sulphate | ≤ 0.1 %
| | Water dissolution test | Eligible
| | Chloride (Cl) | ≤ 0.005%
| | Heavy metal | ≤ 0.06%
| | Standard | Technical grade
| | Capacity | 100MT/month
| | Loading port | Shanghai
| | Use | Selenium dioxide can be used as manganese electrolysis additive, microelement additive for beverage, blackening agent for steal material.Selenium dioxide can be used to deposit zirconium, hafnium and selenium compounds
| | Package | 25kg/drum,or according to the customers’ demand
| | Storage | Stored in the dry and ventilated inside storeroom, prevent direct sunlight, slightly pile and put down |
| Appearance | White crystal
| | Selenium Dioxide | ≥ 98.0 %
| | Hydrochloric acid insoluble | ≤ 0.02 %
| | Fe | ≤ 0.002 %
| | Loss on drying | ≤ 1.0 %
| | Selenate and sulphate | ≤ 0.1 %
| | Water dissolution test | Eligible
| | Chloride (Cl) | ≤ 0.005%
| | Heavy metal | ≤ 0.06%
| | Standard | Technical grade
| | Capacity | 100MT/month
| | Loading port | Shanghai
| | Use | Selenium dioxide can be used as manganese electrolysis additive, microelement additive for beverage, blackening agent for steal material.Selenium dioxide can be used to deposit zirconium, hafnium and selenium compounds
| | Package | 25kg/drum,or according to the customers’ demand
| | Storage | Stored in the dry and ventilated inside storeroom, prevent direct sunlight, slightly pile and put down |
H&Z Industry Co.,Ltd
P.R.China
| Appearance | White crystal
| | Selenium Dioxide | ≥ 98.0 %
| | Hydrochloric acid insoluble | ≤ 0.02 %
| | Fe | ≤ 0.002 %
| | Loss on drying | ≤ 1.0 %
| | Selenate and sulphate | ≤ 0.1 %
| | Water dissolution test | Eligible
| | Chloride (Cl) | ≤ 0.005%
| | Heavy metal | ≤ 0.06%
| | Standard | Technical grade
| | Capacity | 100MT/month
| | Loading port | Shanghai
| | Use | Selenium dioxide can be used as manganese electrolysis additive, microelement additive for beverage, blackening agent for steal material.Selenium dioxide can be used to deposit zirconium, hafnium and selenium compounds
| | Package | 25kg/drum,or according to the customers’ demand
| | Storage | Stored in the dry and ventilated inside storeroom, prevent direct sunlight, slightly pile and put down |
| Appearance | White crystal
| | Selenium Dioxide | ≥ 98.0 %
| | Hydrochloric acid insoluble | ≤ 0.02 %
| | Fe | ≤ 0.002 %
| | Loss on drying | ≤ 1.0 %
| | Selenate and sulphate | ≤ 0.1 %
| | Water dissolution test | Eligible
| | Chloride (Cl) | ≤ 0.005%
| | Heavy metal | ≤ 0.06%
| | Standard | Technical grade
| | Capacity | 100MT/month
| | Loading port | Shanghai
| | Use | Selenium dioxide can be used as manganese electrolysis additive, microelement additive for beverage, blackening agent for steal material.Selenium dioxide can be used to deposit zirconium, hafnium and selenium compounds
| | Package | 25kg/drum,or according to the customers’ demand
| | Storage | Stored in the dry and ventilated inside storeroom, prevent direct sunlight, slightly pile and put down |
Xingrui Industry Co., Limited
P.R.China
| Appearance | White crystal
| | Selenium Dioxide | ≥ 98.0 %
| | Hydrochloric acid insoluble | ≤ 0.02 %
| | Fe | ≤ 0.002 %
| | Loss on drying | ≤ 1.0 %
| | Selenate and sulphate | ≤ 0.1 %
| | Water dissolution test | Eligible
| | Chloride (Cl) | ≤ 0.005%
| | Heavy metal | ≤ 0.06%
| | Standard | Technical grade
| | Capacity | 100MT/month
| | Loading port | Shanghai
| | Use | Selenium dioxide can be used as manganese electrolysis additive, microelement additive for beverage, blackening agent for steal material.Selenium dioxide can be used to deposit zirconium, hafnium and selenium compounds
| | Package | 25kg/drum,or according to the customers’ demand
| | Storage | Stored in the dry and ventilated inside storeroom, prevent direct sunlight, slightly pile and put down |
Hangzhou Meite Industry Co., Ltd (Hangzhou Meite Chemical Co., Ltd)
P.R.China
| Appearance | White crystal
| | Selenium Dioxide | ≥ 98.0 %
| | Hydrochloric acid insoluble | ≤ 0.02 %
| | Fe | ≤ 0.002 %
| | Loss on drying | ≤ 1.0 %
| | Selenate and sulphate | ≤ 0.1 %
| | Water dissolution test | Eligible
| | Chloride (Cl) | ≤ 0.005%
| | Heavy metal | ≤ 0.06%
| | Standard | Technical grade
| | Capacity | 100MT/month
| | Loading port | Shanghai
| | Use | Selenium dioxide can be used as manganese electrolysis additive, microelement additive for beverage, blackening agent for steal material.Selenium dioxide can be used to deposit zirconium, hafnium and selenium compounds
| | Package | 25kg/drum,or according to the customers’ demand
| | Storage | Stored in the dry and ventilated inside storeroom, prevent direct sunlight, slightly pile and put down |
Leap Chem Co., Ltd
P.R.China
Molecular Formula: O2Se
More details are to be found here
ProChem, Inc.
USA
Molecular Formula: O2Se
More details are to be found here
Refine Chemical Co.,Ltd.
P.R.China
Molecular Formula: O2Se
More details are to be found here
OPQ Chemical Co., Ltd
P.R.China
Molecular Formula: O2Se
More details are to be found here
Create Chemical Co., Ltd.
P.R.China
Molecular Formula: O2Se
More details are to be found here
Anderson & Steinssen Inc
USA
Molecular Formula: O2Se
More details are to be found here
Shandong Lanhai Industry Co.,Ltd.
P.R.China
Molecular Formula: O2Se
More details are to be found here
Krada CPS Industry S.L
Spain
Molecular Formula: O2Se
More details are to be found here
Shandong Minglang Chemical Co., Ltd
P.R.China
Molecular Formula: O2Se
More details are to be found here
Fox Chemicals GmbH
Germany
Molecular Formula: O2Se
More details are to be found here
Lori Industry Co., Ltd
P.R.China
Molecular Formula: O2Se
More details are to be found here
Zehao Industry Co., Ltd.
P.R.China
| Property | white crystal powder
| | Selenium Content | ≥ 69% |
Shandong SanYoung Industry Co., Ltd
P.R.China
| Property | white crystal powder
| | Selenium Content | ≥ 69% |
Xiamen Equation Chemical Co.,Ltd
P.R.China
| Property | white crystal powder
| | Selenium Content | ≥ 69% |
AK Scientific, Inc.
USA
| Property | white crystal powder
| | Selenium Content | ≥ 69% |
Chemos GmbH & Co. KG
Germany
| Property | white crystal powder
| | Selenium Content | ≥ 69% |
BuGuCh & Partners
Germany
Selenium Dioxide 98%min
Selenium dioxide Basic information
Product Name: Selenium dioxide
Synonyms: Selenious acid anhydride;SELENIOUS ANHYDRIDE;SELENIUM AA/ICP CALIBRATION/CHECK STANDARD;SELENIUM AA SINGLE ELEMENT STANDARD;SELENIUM, AAS STANDARD SOLUTION;SELENIUM AA STANDARD;SELENIUM ATOMIC ABSORPTION SINGLE ELEMENT STANDARD;SELENIUM ATOMIC ABSORPTION STANDARD
CAS: 7446-08-4
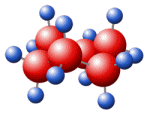
MF: O2Se
MW: 110.96
EINECS: 231-957-4
Product Categories: Inorganics;Metal and Ceramic Science;Oxides;Selenium;Others;Oxidation;Synthetic Reagents;metal oxide
Mol File: 7446-08-4.mol
Selenium dioxide Chemical Properties
mp 315 °C (subl.)(lit.)
bp 684.9 °C(lit.)
density 4.81 g/mL at 25 °C(lit.)
vapor pressure 1 mm Hg ( 157 °C)
Fp 315°C
solubility H2O: soluble
form powder
color white
Water Solubility 38.4 g/100 mL (14 ºC)
Sublimation 315 ºC
Merck 14,8434
Stability: Stable. Incompatible with organic materials, strong acids, ammonia, nitric acid, halogen acids. Protect from moisture.
CAS DataBase Reference 7446-08-4(CAS DataBase Reference)
NIST Chemistry Reference Selenium dioxide(7446-08-4)
EPA Substance Registry System Selenium oxide (SeO2)(7446-08-4)
Selenium dioxide Usage And Synthesis
Chemical Properties yellowish white to reddish powder or crystals
General Description A white or creamy-white volatile lustrous crystal or crystalline powder with a pungent sour smell. Melting point 340 deg C. Density 3.954 g / cm3 . Toxic by ingestion and inhalation.
Air & Water Reactions In presence of water will corrode most metals [USCG, 1999]. Readily soluble in water forming selenious (selenous) acid.
Reactivity Profile Inorganic oxidizing agents, such as Selenium dioxide , react with reducing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. Selenium dioxide reacts with water, particularly hot water, to give selenious (selenous) acid, a weak acid that is corrosive. Stable to light and heat. Rapidly absorbs dry hydrogen fluoride, hydrogen chloride, hydrogen bromide to form the corresponding selenium oxohalide. A good oxidizing agent. Reacts oxidatively with ammonia to form dinitrogen gas and selenium [Merck]. Oxidizes many organic substances.
Health Hazard Absorption of selenium may be demonstrated by presence of the element in the urine and by a garlic-like odor of the breath. Inhalation of dust can cause bronchial spasms, symptoms of asphyxiation, and pneumonitis. Acute symptoms of ingestion include sternal pain, cough, nausea, pallor, coated tongue, gastrointestinal disorders, nervousness, and conjunctivitis. Contact with eyes causes irritation.
Fire Hazard Special Hazards of Combustion Products: Sublimes and forms toxic vapors when heated in fire.
Raw materials Nitric acid--> Oxygen--> Selenium--> NITROGEN DIOXIDE
RETORTE GmbH
Germany
Selenium Dioxide 98%min
Selenium dioxide Basic information
Product Name: Selenium dioxide
Synonyms: Selenious acid anhydride;SELENIOUS ANHYDRIDE;SELENIUM AA/ICP CALIBRATION/CHECK STANDARD;SELENIUM AA SINGLE ELEMENT STANDARD;SELENIUM, AAS STANDARD SOLUTION;SELENIUM AA STANDARD;SELENIUM ATOMIC ABSORPTION SINGLE ELEMENT STANDARD;SELENIUM ATOMIC ABSORPTION STANDARD
CAS: 7446-08-4
MF: O2Se
MW: 110.96
EINECS: 231-957-4
Product Categories: Inorganics;Metal and Ceramic Science;Oxides;Selenium;Others;Oxidation;Synthetic Reagents;metal oxide
Mol File: 7446-08-4.mol
Selenium dioxide Chemical Properties
mp 315 °C (subl.)(lit.)
bp 684.9 °C(lit.)
density 4.81 g/mL at 25 °C(lit.)
vapor pressure 1 mm Hg ( 157 °C)
Fp 315°C
solubility H2O: soluble
form powder
color white
Water Solubility 38.4 g/100 mL (14 ºC)
Sublimation 315 ºC
Merck 14,8434
Stability: Stable. Incompatible with organic materials, strong acids, ammonia, nitric acid, halogen acids. Protect from moisture.
CAS DataBase Reference 7446-08-4(CAS DataBase Reference)
NIST Chemistry Reference Selenium dioxide(7446-08-4)
EPA Substance Registry System Selenium oxide (SeO2)(7446-08-4)
Selenium dioxide Usage And Synthesis
Chemical Properties yellowish white to reddish powder or crystals
General Description A white or creamy-white volatile lustrous crystal or crystalline powder with a pungent sour smell. Melting point 340 deg C. Density 3.954 g / cm3 . Toxic by ingestion and inhalation.
Air & Water Reactions In presence of water will corrode most metals [USCG, 1999]. Readily soluble in water forming selenious (selenous) acid.
Reactivity Profile Inorganic oxidizing agents, such as Selenium dioxide , react with reducing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. Selenium dioxide reacts with water, particularly hot water, to give selenious (selenous) acid, a weak acid that is corrosive. Stable to light and heat. Rapidly absorbs dry hydrogen fluoride, hydrogen chloride, hydrogen bromide to form the corresponding selenium oxohalide. A good oxidizing agent. Reacts oxidatively with ammonia to form dinitrogen gas and selenium [Merck]. Oxidizes many organic substances.
Health Hazard Absorption of selenium may be demonstrated by presence of the element in the urine and by a garlic-like odor of the breath. Inhalation of dust can cause bronchial spasms, symptoms of asphyxiation, and pneumonitis. Acute symptoms of ingestion include sternal pain, cough, nausea, pallor, coated tongue, gastrointestinal disorders, nervousness, and conjunctivitis. Contact with eyes causes irritation.
Fire Hazard Special Hazards of Combustion Products: Sublimes and forms toxic vapors when heated in fire.
Raw materials Nitric acid--> Oxygen--> Selenium--> NITROGEN DIOXIDE
United Mineral & Chemical Corp.
USA
Selenium Dioxide 98%min
Selenium dioxide Basic information
Product Name: Selenium dioxide
Synonyms: Selenious acid anhydride;SELENIOUS ANHYDRIDE;SELENIUM AA/ICP CALIBRATION/CHECK STANDARD;SELENIUM AA SINGLE ELEMENT STANDARD;SELENIUM, AAS STANDARD SOLUTION;SELENIUM AA STANDARD;SELENIUM ATOMIC ABSORPTION SINGLE ELEMENT STANDARD;SELENIUM ATOMIC ABSORPTION STANDARD
CAS: 7446-08-4
MF: O2Se
MW: 110.96
EINECS: 231-957-4
Product Categories: Inorganics;Metal and Ceramic Science;Oxides;Selenium;Others;Oxidation;Synthetic Reagents;metal oxide
Mol File: 7446-08-4.mol
Selenium dioxide Chemical Properties
mp 315 °C (subl.)(lit.)
bp 684.9 °C(lit.)
density 4.81 g/mL at 25 °C(lit.)
vapor pressure 1 mm Hg ( 157 °C)
Fp 315°C
solubility H2O: soluble
form powder
color white
Water Solubility 38.4 g/100 mL (14 ºC)
Sublimation 315 ºC
Merck 14,8434
Stability: Stable. Incompatible with organic materials, strong acids, ammonia, nitric acid, halogen acids. Protect from moisture.
CAS DataBase Reference 7446-08-4(CAS DataBase Reference)
NIST Chemistry Reference Selenium dioxide(7446-08-4)
EPA Substance Registry System Selenium oxide (SeO2)(7446-08-4)
Selenium dioxide Usage And Synthesis
Chemical Properties yellowish white to reddish powder or crystals
General Description A white or creamy-white volatile lustrous crystal or crystalline powder with a pungent sour smell. Melting point 340 deg C. Density 3.954 g / cm3 . Toxic by ingestion and inhalation.
Air & Water Reactions In presence of water will corrode most metals [USCG, 1999]. Readily soluble in water forming selenious (selenous) acid.
Reactivity Profile Inorganic oxidizing agents, such as Selenium dioxide , react with reducing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. Selenium dioxide reacts with water, particularly hot water, to give selenious (selenous) acid, a weak acid that is corrosive. Stable to light and heat. Rapidly absorbs dry hydrogen fluoride, hydrogen chloride, hydrogen bromide to form the corresponding selenium oxohalide. A good oxidizing agent. Reacts oxidatively with ammonia to form dinitrogen gas and selenium [Merck]. Oxidizes many organic substances.
Health Hazard Absorption of selenium may be demonstrated by presence of the element in the urine and by a garlic-like odor of the breath. Inhalation of dust can cause bronchial spasms, symptoms of asphyxiation, and pneumonitis. Acute symptoms of ingestion include sternal pain, cough, nausea, pallor, coated tongue, gastrointestinal disorders, nervousness, and conjunctivitis. Contact with eyes causes irritation.
Fire Hazard Special Hazards of Combustion Products: Sublimes and forms toxic vapors when heated in fire.
Raw materials Nitric acid--> Oxygen--> Selenium--> NITROGEN DIOXIDE
Atlantic Equipment Engineers, Div. Micron Metals, Inc.
USA
Selenium Dioxide 98%min
Selenium dioxide Basic information
Product Name: Selenium dioxide
Synonyms: Selenious acid anhydride;SELENIOUS ANHYDRIDE;SELENIUM AA/ICP CALIBRATION/CHECK STANDARD;SELENIUM AA SINGLE ELEMENT STANDARD;SELENIUM, AAS STANDARD SOLUTION;SELENIUM AA STANDARD;SELENIUM ATOMIC ABSORPTION SINGLE ELEMENT STANDARD;SELENIUM ATOMIC ABSORPTION STANDARD
CAS: 7446-08-4
MF: O2Se
MW: 110.96
EINECS: 231-957-4
Product Categories: Inorganics;Metal and Ceramic Science;Oxides;Selenium;Others;Oxidation;Synthetic Reagents;metal oxide
Mol File: 7446-08-4.mol
Selenium dioxide Chemical Properties
mp 315 °C (subl.)(lit.)
bp 684.9 °C(lit.)
density 4.81 g/mL at 25 °C(lit.)
vapor pressure 1 mm Hg ( 157 °C)
Fp 315°C
solubility H2O: soluble
form powder
color white
Water Solubility 38.4 g/100 mL (14 ºC)
Sublimation 315 ºC
Merck 14,8434
Stability: Stable. Incompatible with organic materials, strong acids, ammonia, nitric acid, halogen acids. Protect from moisture.
CAS DataBase Reference 7446-08-4(CAS DataBase Reference)
NIST Chemistry Reference Selenium dioxide(7446-08-4)
EPA Substance Registry System Selenium oxide (SeO2)(7446-08-4)
Selenium dioxide Usage And Synthesis
Chemical Properties yellowish white to reddish powder or crystals
General Description A white or creamy-white volatile lustrous crystal or crystalline powder with a pungent sour smell. Melting point 340 deg C. Density 3.954 g / cm3 . Toxic by ingestion and inhalation.
Air & Water Reactions In presence of water will corrode most metals [USCG, 1999]. Readily soluble in water forming selenious (selenous) acid.
Reactivity Profile Inorganic oxidizing agents, such as Selenium dioxide , react with reducing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. Selenium dioxide reacts with water, particularly hot water, to give selenious (selenous) acid, a weak acid that is corrosive. Stable to light and heat. Rapidly absorbs dry hydrogen fluoride, hydrogen chloride, hydrogen bromide to form the corresponding selenium oxohalide. A good oxidizing agent. Reacts oxidatively with ammonia to form dinitrogen gas and selenium [Merck]. Oxidizes many organic substances.
Health Hazard Absorption of selenium may be demonstrated by presence of the element in the urine and by a garlic-like odor of the breath. Inhalation of dust can cause bronchial spasms, symptoms of asphyxiation, and pneumonitis. Acute symptoms of ingestion include sternal pain, cough, nausea, pallor, coated tongue, gastrointestinal disorders, nervousness, and conjunctivitis. Contact with eyes causes irritation.
Fire Hazard Special Hazards of Combustion Products: Sublimes and forms toxic vapors when heated in fire.
Raw materials Nitric acid--> Oxygen--> Selenium--> NITROGEN DIOXIDE
Merck Schuchardt OHG
Germany
Selenium Dioxide 98%min
Selenium dioxide Basic information
Product Name: Selenium dioxide
Synonyms: Selenious acid anhydride;SELENIOUS ANHYDRIDE;SELENIUM AA/ICP CALIBRATION/CHECK STANDARD;SELENIUM AA SINGLE ELEMENT STANDARD;SELENIUM, AAS STANDARD SOLUTION;SELENIUM AA STANDARD;SELENIUM ATOMIC ABSORPTION SINGLE ELEMENT STANDARD;SELENIUM ATOMIC ABSORPTION STANDARD
CAS: 7446-08-4
MF: O2Se
MW: 110.96
EINECS: 231-957-4
Product Categories: Inorganics;Metal and Ceramic Science;Oxides;Selenium;Others;Oxidation;Synthetic Reagents;metal oxide
Mol File: 7446-08-4.mol
Selenium dioxide Chemical Properties
mp 315 °C (subl.)(lit.)
bp 684.9 °C(lit.)
density 4.81 g/mL at 25 °C(lit.)
vapor pressure 1 mm Hg ( 157 °C)
Fp 315°C
solubility H2O: soluble
form powder
color white
Water Solubility 38.4 g/100 mL (14 ºC)
Sublimation 315 ºC
Merck 14,8434
Stability: Stable. Incompatible with organic materials, strong acids, ammonia, nitric acid, halogen acids. Protect from moisture.
CAS DataBase Reference 7446-08-4(CAS DataBase Reference)
NIST Chemistry Reference Selenium dioxide(7446-08-4)
EPA Substance Registry System Selenium oxide (SeO2)(7446-08-4)
Selenium dioxide Usage And Synthesis
Chemical Properties yellowish white to reddish powder or crystals
General Description A white or creamy-white volatile lustrous crystal or crystalline powder with a pungent sour smell. Melting point 340 deg C. Density 3.954 g / cm3 . Toxic by ingestion and inhalation.
Air & Water Reactions In presence of water will corrode most metals [USCG, 1999]. Readily soluble in water forming selenious (selenous) acid.
Reactivity Profile Inorganic oxidizing agents, such as Selenium dioxide , react with reducing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. Selenium dioxide reacts with water, particularly hot water, to give selenious (selenous) acid, a weak acid that is corrosive. Stable to light and heat. Rapidly absorbs dry hydrogen fluoride, hydrogen chloride, hydrogen bromide to form the corresponding selenium oxohalide. A good oxidizing agent. Reacts oxidatively with ammonia to form dinitrogen gas and selenium [Merck]. Oxidizes many organic substances.
Health Hazard Absorption of selenium may be demonstrated by presence of the element in the urine and by a garlic-like odor of the breath. Inhalation of dust can cause bronchial spasms, symptoms of asphyxiation, and pneumonitis. Acute symptoms of ingestion include sternal pain, cough, nausea, pallor, coated tongue, gastrointestinal disorders, nervousness, and conjunctivitis. Contact with eyes causes irritation.
Fire Hazard Special Hazards of Combustion Products: Sublimes and forms toxic vapors when heated in fire.
Raw materials Nitric acid--> Oxygen--> Selenium--> NITROGEN DIOXIDE
Santa Cruz Biotechnology, Inc.
USA
Selenium Dioxide 98%min
Selenium dioxide Basic information
Product Name: Selenium dioxide
Synonyms: Selenious acid anhydride;SELENIOUS ANHYDRIDE;SELENIUM AA/ICP CALIBRATION/CHECK STANDARD;SELENIUM AA SINGLE ELEMENT STANDARD;SELENIUM, AAS STANDARD SOLUTION;SELENIUM AA STANDARD;SELENIUM ATOMIC ABSORPTION SINGLE ELEMENT STANDARD;SELENIUM ATOMIC ABSORPTION STANDARD
CAS: 7446-08-4
MF: O2Se
MW: 110.96
EINECS: 231-957-4
Product Categories: Inorganics;Metal and Ceramic Science;Oxides;Selenium;Others;Oxidation;Synthetic Reagents;metal oxide
Mol File: 7446-08-4.mol
Selenium dioxide Chemical Properties
mp 315 °C (subl.)(lit.)
bp 684.9 °C(lit.)
density 4.81 g/mL at 25 °C(lit.)
vapor pressure 1 mm Hg ( 157 °C)
Fp 315°C
solubility H2O: soluble
form powder
color white
Water Solubility 38.4 g/100 mL (14 ºC)
Sublimation 315 ºC
Merck 14,8434
Stability: Stable. Incompatible with organic materials, strong acids, ammonia, nitric acid, halogen acids. Protect from moisture.
CAS DataBase Reference 7446-08-4(CAS DataBase Reference)
NIST Chemistry Reference Selenium dioxide(7446-08-4)
EPA Substance Registry System Selenium oxide (SeO2)(7446-08-4)
Selenium dioxide Usage And Synthesis
Chemical Properties yellowish white to reddish powder or crystals
General Description A white or creamy-white volatile lustrous crystal or crystalline powder with a pungent sour smell. Melting point 340 deg C. Density 3.954 g / cm3 . Toxic by ingestion and inhalation.
Air & Water Reactions In presence of water will corrode most metals [USCG, 1999]. Readily soluble in water forming selenious (selenous) acid.
Reactivity Profile Inorganic oxidizing agents, such as Selenium dioxide , react with reducing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. Selenium dioxide reacts with water, particularly hot water, to give selenious (selenous) acid, a weak acid that is corrosive. Stable to light and heat. Rapidly absorbs dry hydrogen fluoride, hydrogen chloride, hydrogen bromide to form the corresponding selenium oxohalide. A good oxidizing agent. Reacts oxidatively with ammonia to form dinitrogen gas and selenium [Merck]. Oxidizes many organic substances.
Health Hazard Absorption of selenium may be demonstrated by presence of the element in the urine and by a garlic-like odor of the breath. Inhalation of dust can cause bronchial spasms, symptoms of asphyxiation, and pneumonitis. Acute symptoms of ingestion include sternal pain, cough, nausea, pallor, coated tongue, gastrointestinal disorders, nervousness, and conjunctivitis. Contact with eyes causes irritation.
Fire Hazard Special Hazards of Combustion Products: Sublimes and forms toxic vapors when heated in fire.
Raw materials Nitric acid--> Oxygen--> Selenium--> NITROGEN DIOXIDE
|

 details
details details
details details
details


_Co_Ltd_1_575.jpg)





