|
Suppliers for CAS
97-63-2
|
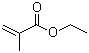
Properties | | CAS |
97-63-2 | | Formula |
C6H10O2 | | EINECS |
202-597-5 |
|
|
23 Registered suppliers
Simagchem Corporation
P.R.China
Aecochem Corp.
P.R.China
Dayang Chem (Hangzhou) Co.,Ltd.
P.R.China
Sancai Industry Co.,Ltd.
P.R.China
H&Z Industry Co.,Ltd
P.R.China
Xingrui Industry Co., Limited
P.R.China
Hangzhou Meite Industry Co., Ltd (Hangzhou Meite Chemical Co., Ltd)
P.R.China
Leap Chem Co., Ltd
P.R.China
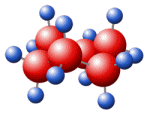
Molecular Formula: C6H10O2
Molecular Weight: 114.14
Hazard Symbols: F,Xi
UN Number: UN 2277 3/PG 2
WGKGermany: 1
PackingGroup: II
HS Code: 29161490
BCH Brühl - Chemikalien Handel GmbH
Germany
Molecular Formula: C6H10O2
Molecular Weight: 114.14
Hazard Symbols: F,Xi
UN Number: UN 2277 3/PG 2
WGKGermany: 1
PackingGroup: II
HS Code: 29161490
Molecular Formula: C6H10O2
Molecular Weight: 114.14
Hazard Symbols: F,Xi
UN Number: UN 2277 3/PG 2
WGKGermany: 1
PackingGroup: II
HS Code: 29161490
Fountain Biotechnology Co.,Ltd
P.R.China
Molecular Formula: C6H10O2
Molecular Weight: 114.14
Hazard Symbols: F,Xi
UN Number: UN 2277 3/PG 2
WGKGermany: 1
PackingGroup: II
HS Code: 29161490
Refine Chemical Co.,Ltd.
P.R.China
Molecular Formula: C6H10O2
Molecular Weight: 114.14
Hazard Symbols: F,Xi
UN Number: UN 2277 3/PG 2
WGKGermany: 1
PackingGroup: II
HS Code: 29161490
OPQ Chemical Co., Ltd
P.R.China
Molecular Formula: C6H10O2
Molecular Weight: 114.14
Hazard Symbols: F,Xi
UN Number: UN 2277 3/PG 2
WGKGermany: 1
PackingGroup: II
HS Code: 29161490
Lori Industry Co., Ltd
P.R.China
Molecular Formula: C6H10O2
Molecular Weight: 114.14
Hazard Symbols: F,Xi
UN Number: UN 2277 3/PG 2
WGKGermany: 1
PackingGroup: II
HS Code: 29161490
Shandong SanYoung Industry Co., Ltd
P.R.China
Molecular Formula: C6H10O2
Molecular Weight: 114.14
Hazard Symbols: F,Xi
UN Number: UN 2277 3/PG 2
WGKGermany: 1
PackingGroup: II
HS Code: 29161490
Xiamen Equation Chemical Co.,Ltd
P.R.China
Molecular Formula: C6H10O2
Molecular Weight: 114.14
Hazard Symbols: F,Xi
UN Number: UN 2277 3/PG 2
WGKGermany: 1
PackingGroup: II
HS Code: 29161490
Chemos GmbH & Co. KG
Germany
Molecular Formula: C6H10O2
Molecular Weight: 114.14
Hazard Symbols: F,Xi
UN Number: UN 2277 3/PG 2
WGKGermany: 1
PackingGroup: II
HS Code: 29161490
BuGuCh & Partners
Germany
Product Name: Ethyl methacrylate
Synonyms: 2-Methyl-2-propenoic acid ethyl ester;2-methyl-2-propenoicacid,ethylester;2-methyl-2-propenoicaciethylester;2-Methylacrylic acid, ethyl ester;2-methyl-prop-2-enoicacidethylester;2-Propenoicacid,2-methyl-,ethylester;EMA;Ethyl 2-methacrylate
CAS: 97-63-2
MF: C6H10O2
MW: 114.14
EINECS: 202-597-5
mp -75 °C
bp 118-119 °C(lit.)
density 0.917 g/mL at 25 °C(lit.)
vapor density > 3.9 (vs air)
vapor pressure 15 mm Hg ( 20 °C)
refractive index n20/D 1.413(lit.)
Fp 60 °F
storage temp. Refrigerator (+4°C) + Flammables area
Water Solubility 4 g/L (20 ºC)
BRN 471201
Ethyl methacrylate Usage And Synthesis
Chemical Properties colourless liquid with an unpleasant odour
General Description A colorless moderately toxic liquid with an acrid odor. Flash point of 70°F. Boiling point 278°F. Vapors irritate the eyes and respiratory system. Less dense than water. Not soluble in water. Used to make polymers and other chemicals.
Air & Water Reactions Highly flammable. A very dangerous fire and explosion hazard. Not soluble in water.
Reactivity Profile May polymerize if heated for prolonged periods or accidentally contaminated. If polymerization takes place inside a container, the container may violently rupture. Can react with oxidizing materials. When heated to decomposition Ethyl methacrylate emits irritating fumes and acrid smoke [Sax, 9th ed., 1996, p. 1576].
Health Hazard Inhalation may cause irritation of the mucous membrane. Ingestion causes irritation of mouth and stomach. Contact with liquid irritates eyes and skin.
Fire Hazard Behavior in Fire: Sealed containers may rupture explosively if hot. Heat can cause a violent polymerization reaction with rapid release of energy. Vapors are heavier than air and can travel to a source of ignition and flash back.
Ethyl methacrylate Preparation Products And Raw materials
Raw materials Methacrylic acid--> Acetone cyanohydrin --> Methacrylamide
Preparation Products Procymidone--> Leather lustering agent
Product Name: Ethyl methacrylate
Synonyms: 2-Methyl-2-propenoic acid ethyl ester;2-methyl-2-propenoicacid,ethylester;2-methyl-2-propenoicaciethylester;2-Methylacrylic acid, ethyl ester;2-methyl-prop-2-enoicacidethylester;2-Propenoicacid,2-methyl-,ethylester;EMA;Ethyl 2-methacrylate
CAS: 97-63-2
MF: C6H10O2
MW: 114.14
EINECS: 202-597-5
mp -75 °C
bp 118-119 °C(lit.)
density 0.917 g/mL at 25 °C(lit.)
vapor density > 3.9 (vs air)
vapor pressure 15 mm Hg ( 20 °C)
refractive index n20/D 1.413(lit.)
Fp 60 °F
storage temp. Refrigerator (+4°C) + Flammables area
Water Solubility 4 g/L (20 ºC)
BRN 471201
Ethyl methacrylate Usage And Synthesis
Chemical Properties colourless liquid with an unpleasant odour
General Description A colorless moderately toxic liquid with an acrid odor. Flash point of 70°F. Boiling point 278°F. Vapors irritate the eyes and respiratory system. Less dense than water. Not soluble in water. Used to make polymers and other chemicals.
Air & Water Reactions Highly flammable. A very dangerous fire and explosion hazard. Not soluble in water.
Reactivity Profile May polymerize if heated for prolonged periods or accidentally contaminated. If polymerization takes place inside a container, the container may violently rupture. Can react with oxidizing materials. When heated to decomposition Ethyl methacrylate emits irritating fumes and acrid smoke [Sax, 9th ed., 1996, p. 1576].
Health Hazard Inhalation may cause irritation of the mucous membrane. Ingestion causes irritation of mouth and stomach. Contact with liquid irritates eyes and skin.
Fire Hazard Behavior in Fire: Sealed containers may rupture explosively if hot. Heat can cause a violent polymerization reaction with rapid release of energy. Vapors are heavier than air and can travel to a source of ignition and flash back.
Ethyl methacrylate Preparation Products And Raw materials
Raw materials Methacrylic acid--> Acetone cyanohydrin --> Methacrylamide
Preparation Products Procymidone--> Leather lustering agent
Ring Specialty Chemicals Inc.
Canada
Product Name: Ethyl methacrylate
Synonyms: 2-Methyl-2-propenoic acid ethyl ester;2-methyl-2-propenoicacid,ethylester;2-methyl-2-propenoicaciethylester;2-Methylacrylic acid, ethyl ester;2-methyl-prop-2-enoicacidethylester;2-Propenoicacid,2-methyl-,ethylester;EMA;Ethyl 2-methacrylate
CAS: 97-63-2
MF: C6H10O2
MW: 114.14
EINECS: 202-597-5
mp -75 °C
bp 118-119 °C(lit.)
density 0.917 g/mL at 25 °C(lit.)
vapor density > 3.9 (vs air)
vapor pressure 15 mm Hg ( 20 °C)
refractive index n20/D 1.413(lit.)
Fp 60 °F
storage temp. Refrigerator (+4°C) + Flammables area
Water Solubility 4 g/L (20 ºC)
BRN 471201
Ethyl methacrylate Usage And Synthesis
Chemical Properties colourless liquid with an unpleasant odour
General Description A colorless moderately toxic liquid with an acrid odor. Flash point of 70°F. Boiling point 278°F. Vapors irritate the eyes and respiratory system. Less dense than water. Not soluble in water. Used to make polymers and other chemicals.
Air & Water Reactions Highly flammable. A very dangerous fire and explosion hazard. Not soluble in water.
Reactivity Profile May polymerize if heated for prolonged periods or accidentally contaminated. If polymerization takes place inside a container, the container may violently rupture. Can react with oxidizing materials. When heated to decomposition Ethyl methacrylate emits irritating fumes and acrid smoke [Sax, 9th ed., 1996, p. 1576].
Health Hazard Inhalation may cause irritation of the mucous membrane. Ingestion causes irritation of mouth and stomach. Contact with liquid irritates eyes and skin.
Fire Hazard Behavior in Fire: Sealed containers may rupture explosively if hot. Heat can cause a violent polymerization reaction with rapid release of energy. Vapors are heavier than air and can travel to a source of ignition and flash back.
Ethyl methacrylate Preparation Products And Raw materials
Raw materials Methacrylic acid--> Acetone cyanohydrin --> Methacrylamide
Preparation Products Procymidone--> Leather lustering agent
World. Chem. Co., Ltd.
P.R.China
Product Name: Ethyl methacrylate
Synonyms: 2-Methyl-2-propenoic acid ethyl ester;2-methyl-2-propenoicacid,ethylester;2-methyl-2-propenoicaciethylester;2-Methylacrylic acid, ethyl ester;2-methyl-prop-2-enoicacidethylester;2-Propenoicacid,2-methyl-,ethylester;EMA;Ethyl 2-methacrylate
CAS: 97-63-2
MF: C6H10O2
MW: 114.14
EINECS: 202-597-5
mp -75 °C
bp 118-119 °C(lit.)
density 0.917 g/mL at 25 °C(lit.)
vapor density > 3.9 (vs air)
vapor pressure 15 mm Hg ( 20 °C)
refractive index n20/D 1.413(lit.)
Fp 60 °F
storage temp. Refrigerator (+4°C) + Flammables area
Water Solubility 4 g/L (20 ºC)
BRN 471201
Ethyl methacrylate Usage And Synthesis
Chemical Properties colourless liquid with an unpleasant odour
General Description A colorless moderately toxic liquid with an acrid odor. Flash point of 70°F. Boiling point 278°F. Vapors irritate the eyes and respiratory system. Less dense than water. Not soluble in water. Used to make polymers and other chemicals.
Air & Water Reactions Highly flammable. A very dangerous fire and explosion hazard. Not soluble in water.
Reactivity Profile May polymerize if heated for prolonged periods or accidentally contaminated. If polymerization takes place inside a container, the container may violently rupture. Can react with oxidizing materials. When heated to decomposition Ethyl methacrylate emits irritating fumes and acrid smoke [Sax, 9th ed., 1996, p. 1576].
Health Hazard Inhalation may cause irritation of the mucous membrane. Ingestion causes irritation of mouth and stomach. Contact with liquid irritates eyes and skin.
Fire Hazard Behavior in Fire: Sealed containers may rupture explosively if hot. Heat can cause a violent polymerization reaction with rapid release of energy. Vapors are heavier than air and can travel to a source of ignition and flash back.
Ethyl methacrylate Preparation Products And Raw materials
Raw materials Methacrylic acid--> Acetone cyanohydrin --> Methacrylamide
Preparation Products Procymidone--> Leather lustering agent
Evonik Corporation
USA
Product Name: Ethyl methacrylate
Synonyms: 2-Methyl-2-propenoic acid ethyl ester;2-methyl-2-propenoicacid,ethylester;2-methyl-2-propenoicaciethylester;2-Methylacrylic acid, ethyl ester;2-methyl-prop-2-enoicacidethylester;2-Propenoicacid,2-methyl-,ethylester;EMA;Ethyl 2-methacrylate
CAS: 97-63-2
MF: C6H10O2
MW: 114.14
EINECS: 202-597-5
mp -75 °C
bp 118-119 °C(lit.)
density 0.917 g/mL at 25 °C(lit.)
vapor density > 3.9 (vs air)
vapor pressure 15 mm Hg ( 20 °C)
refractive index n20/D 1.413(lit.)
Fp 60 °F
storage temp. Refrigerator (+4°C) + Flammables area
Water Solubility 4 g/L (20 ºC)
BRN 471201
Ethyl methacrylate Usage And Synthesis
Chemical Properties colourless liquid with an unpleasant odour
General Description A colorless moderately toxic liquid with an acrid odor. Flash point of 70°F. Boiling point 278°F. Vapors irritate the eyes and respiratory system. Less dense than water. Not soluble in water. Used to make polymers and other chemicals.
Air & Water Reactions Highly flammable. A very dangerous fire and explosion hazard. Not soluble in water.
Reactivity Profile May polymerize if heated for prolonged periods or accidentally contaminated. If polymerization takes place inside a container, the container may violently rupture. Can react with oxidizing materials. When heated to decomposition Ethyl methacrylate emits irritating fumes and acrid smoke [Sax, 9th ed., 1996, p. 1576].
Health Hazard Inhalation may cause irritation of the mucous membrane. Ingestion causes irritation of mouth and stomach. Contact with liquid irritates eyes and skin.
Fire Hazard Behavior in Fire: Sealed containers may rupture explosively if hot. Heat can cause a violent polymerization reaction with rapid release of energy. Vapors are heavier than air and can travel to a source of ignition and flash back.
Ethyl methacrylate Preparation Products And Raw materials
Raw materials Methacrylic acid--> Acetone cyanohydrin --> Methacrylamide
Preparation Products Procymidone--> Leather lustering agent
DuPont (UK) Ltd.
U.K.
Product Name: Ethyl methacrylate
Synonyms: 2-Methyl-2-propenoic acid ethyl ester;2-methyl-2-propenoicacid,ethylester;2-methyl-2-propenoicaciethylester;2-Methylacrylic acid, ethyl ester;2-methyl-prop-2-enoicacidethylester;2-Propenoicacid,2-methyl-,ethylester;EMA;Ethyl 2-methacrylate
CAS: 97-63-2
MF: C6H10O2
MW: 114.14
EINECS: 202-597-5
mp -75 °C
bp 118-119 °C(lit.)
density 0.917 g/mL at 25 °C(lit.)
vapor density > 3.9 (vs air)
vapor pressure 15 mm Hg ( 20 °C)
refractive index n20/D 1.413(lit.)
Fp 60 °F
storage temp. Refrigerator (+4°C) + Flammables area
Water Solubility 4 g/L (20 ºC)
BRN 471201
Ethyl methacrylate Usage And Synthesis
Chemical Properties colourless liquid with an unpleasant odour
General Description A colorless moderately toxic liquid with an acrid odor. Flash point of 70°F. Boiling point 278°F. Vapors irritate the eyes and respiratory system. Less dense than water. Not soluble in water. Used to make polymers and other chemicals.
Air & Water Reactions Highly flammable. A very dangerous fire and explosion hazard. Not soluble in water.
Reactivity Profile May polymerize if heated for prolonged periods or accidentally contaminated. If polymerization takes place inside a container, the container may violently rupture. Can react with oxidizing materials. When heated to decomposition Ethyl methacrylate emits irritating fumes and acrid smoke [Sax, 9th ed., 1996, p. 1576].
Health Hazard Inhalation may cause irritation of the mucous membrane. Ingestion causes irritation of mouth and stomach. Contact with liquid irritates eyes and skin.
Fire Hazard Behavior in Fire: Sealed containers may rupture explosively if hot. Heat can cause a violent polymerization reaction with rapid release of energy. Vapors are heavier than air and can travel to a source of ignition and flash back.
Ethyl methacrylate Preparation Products And Raw materials
Raw materials Methacrylic acid--> Acetone cyanohydrin --> Methacrylamide
Preparation Products Procymidone--> Leather lustering agent
Merck Schuchardt OHG
Germany
Product Name: Ethyl methacrylate
Synonyms: 2-Methyl-2-propenoic acid ethyl ester;2-methyl-2-propenoicacid,ethylester;2-methyl-2-propenoicaciethylester;2-Methylacrylic acid, ethyl ester;2-methyl-prop-2-enoicacidethylester;2-Propenoicacid,2-methyl-,ethylester;EMA;Ethyl 2-methacrylate
CAS: 97-63-2
MF: C6H10O2
MW: 114.14
EINECS: 202-597-5
mp -75 °C
bp 118-119 °C(lit.)
density 0.917 g/mL at 25 °C(lit.)
vapor density > 3.9 (vs air)
vapor pressure 15 mm Hg ( 20 °C)
refractive index n20/D 1.413(lit.)
Fp 60 °F
storage temp. Refrigerator (+4°C) + Flammables area
Water Solubility 4 g/L (20 ºC)
BRN 471201
Ethyl methacrylate Usage And Synthesis
Chemical Properties colourless liquid with an unpleasant odour
General Description A colorless moderately toxic liquid with an acrid odor. Flash point of 70°F. Boiling point 278°F. Vapors irritate the eyes and respiratory system. Less dense than water. Not soluble in water. Used to make polymers and other chemicals.
Air & Water Reactions Highly flammable. A very dangerous fire and explosion hazard. Not soluble in water.
Reactivity Profile May polymerize if heated for prolonged periods or accidentally contaminated. If polymerization takes place inside a container, the container may violently rupture. Can react with oxidizing materials. When heated to decomposition Ethyl methacrylate emits irritating fumes and acrid smoke [Sax, 9th ed., 1996, p. 1576].
Health Hazard Inhalation may cause irritation of the mucous membrane. Ingestion causes irritation of mouth and stomach. Contact with liquid irritates eyes and skin.
Fire Hazard Behavior in Fire: Sealed containers may rupture explosively if hot. Heat can cause a violent polymerization reaction with rapid release of energy. Vapors are heavier than air and can travel to a source of ignition and flash back.
Ethyl methacrylate Preparation Products And Raw materials
Raw materials Methacrylic acid--> Acetone cyanohydrin --> Methacrylamide
Preparation Products Procymidone--> Leather lustering agent
Santa Cruz Biotechnology, Inc.
USA
Product Name: Ethyl methacrylate
Synonyms: 2-Methyl-2-propenoic acid ethyl ester;2-methyl-2-propenoicacid,ethylester;2-methyl-2-propenoicaciethylester;2-Methylacrylic acid, ethyl ester;2-methyl-prop-2-enoicacidethylester;2-Propenoicacid,2-methyl-,ethylester;EMA;Ethyl 2-methacrylate
CAS: 97-63-2
MF: C6H10O2
MW: 114.14
EINECS: 202-597-5
mp -75 °C
bp 118-119 °C(lit.)
density 0.917 g/mL at 25 °C(lit.)
vapor density > 3.9 (vs air)
vapor pressure 15 mm Hg ( 20 °C)
refractive index n20/D 1.413(lit.)
Fp 60 °F
storage temp. Refrigerator (+4°C) + Flammables area
Water Solubility 4 g/L (20 ºC)
BRN 471201
Ethyl methacrylate Usage And Synthesis
Chemical Properties colourless liquid with an unpleasant odour
General Description A colorless moderately toxic liquid with an acrid odor. Flash point of 70°F. Boiling point 278°F. Vapors irritate the eyes and respiratory system. Less dense than water. Not soluble in water. Used to make polymers and other chemicals.
Air & Water Reactions Highly flammable. A very dangerous fire and explosion hazard. Not soluble in water.
Reactivity Profile May polymerize if heated for prolonged periods or accidentally contaminated. If polymerization takes place inside a container, the container may violently rupture. Can react with oxidizing materials. When heated to decomposition Ethyl methacrylate emits irritating fumes and acrid smoke [Sax, 9th ed., 1996, p. 1576].
Health Hazard Inhalation may cause irritation of the mucous membrane. Ingestion causes irritation of mouth and stomach. Contact with liquid irritates eyes and skin.
Fire Hazard Behavior in Fire: Sealed containers may rupture explosively if hot. Heat can cause a violent polymerization reaction with rapid release of energy. Vapors are heavier than air and can travel to a source of ignition and flash back.
Ethyl methacrylate Preparation Products And Raw materials
Raw materials Methacrylic acid--> Acetone cyanohydrin --> Methacrylamide
Preparation Products Procymidone--> Leather lustering agent
|


 details
details details
details

_Co_Ltd_1_575.jpg)






