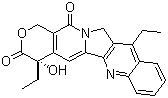
Description:
The main reasons for the superior antitumor activity of 7-Ethylcamptothecin compared with CPT are as follows: (a) 7-Ethylcamptothecin had a stronger growth-inhibiting activity against tumor cells, and (b) 7-Ethylcamptothecin remained in the intestinal tract for a longer time and in higher amounts when administered in vivo. 7-Ethyl Camptothecin is a natural compound found in the barks of Camptotheca acuminata Decne, it can be used as a cosmetics material.
- Molecular Weight: 376.42
- Molecular Formula: C22H20N2O4
Purity: >98%
Canonical SMILES:
CCC1=C2CN3C(=CC4=C(C3=O)COC(=O)C4(CC)O)C2=NC5=CC=CC=C51
InChI:
InChI=1S/C22H20N2O4/c1-3-12-13-7-5-6-8-17(13)23-19-14(12)10-24-18(19)9-16-15(20(24)25)11-28-21(26)22(16,27)4-2/h5-9,27H,3-4,10-11H2,1-2H3/t22-/m0/s1
InChIKey: MYQKIWCVEPUPIL-QFIPXVFZSA-N
- Melting Point: >235°C
- Appearance: Powder
Synonyms:
(S)-4,11-Diethyl-4-hydroxy-1H-pyrano(3',4': 6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione; 7-ethylcamptothecin; (4S)-4,11-diethyl-4-hydroxy-1H-pyrano[3',4': 6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; Irinotecan EP Impurity F
More details are to be found on supplier website