|
|
|

|
Suppliers for
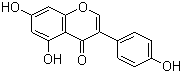
Genistein
|
Properties | | CAS |
446-72-0 | | Formula |
C15H10O5 | | EINECS |
207-174-9 |
|
|
22 Registered suppliers
| Purity(Detect by HPLC) | HPLC≥ 98%
| | Specification | 20mg/branch
| | Molecular Formula | C15H10O5
| | Molecular Weight | 270.24
| | Package | 25kg/drum |
| Purity(Detect by HPLC) | HPLC≥ 98%
| | Specification | 20mg/branch
| | Molecular Formula | C15H10O5
| | Molecular Weight | 270.24
| | Package | 25kg/drum |
| Purity(Detect by HPLC) | HPLC≥ 98%
| | Specification | 20mg/branch
| | Molecular Formula | C15H10O5
| | Molecular Weight | 270.24
| | Package | 25kg/drum |
More details are to be found here
Molecular Formula: C15H10O5
Molecular Weight: 270.24
Hazard Symbols: Xi,Xn
WGKGermany: 3
HS Code: 29329990
Molecular Formula: C15H10O5
Molecular Weight: 270.24
Hazard Symbols: Xi,Xn
WGKGermany: 3
HS Code: 29329990
Molecular Formula: C15H10O5
Molecular Weight: 270.24
Hazard Symbols: Xi,Xn
WGKGermany: 3
HS Code: 29329990
Molecular Formula: C15H10O5
Molecular Weight: 270.24
Hazard Symbols: Xi,Xn
WGKGermany: 3
HS Code: 29329990
Molecular Formula: C15H10O5
Molecular Weight: 270.24
Hazard Symbols: Xi,Xn
WGKGermany: 3
HS Code: 29329990
Molecular Formula: C15H10O5
Molecular Weight: 270.24
Hazard Symbols: Xi,Xn
WGKGermany: 3
HS Code: 29329990
More details are to be found here
More details are to be found here
Description : Genistein, a phytoestrogen found in soy products, is a highly specific inhibitor of protein tyrosine kinase (PTK) which blocks the mitogenic effect mediated by EGF on NIH-3T3 cells with IC50 of 12μM or by insulin with IC50 of 19 μM. Genistein is developed to be an antitumor agent. Genistein has antioxidant effect, it can be used in cosmetics material. - Molecular Weight :270.24
- Boiling Point :555.5±50.0°C at 760 mmHg
- Melting Point :295-300°C
- Purity :> 98%
Molecular Formula : C15H10O5 Canonical SMILES : C1=CC(=CC=C1C2=COC3=CC(=CC(=C3C2=O)O)O)O InChI : InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-13-6-10(17)5-12(18)14(13)15(11)19/h1-7,16-18H
InChIKey :
TZBJGXHYKVUXJN-UHFFFAOYSA-N
Solubility :
Soluble in DMSO (Slightly), Methanol (Slightly)
Appearance :
Pale Yellow to Light Beige Solid
Application :
antitumor agent
Storage : Store at -20°C under inert atmosphere
Synonyms :
5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; Baichanin A; Bonistein; 4',5,7-Trihydroxyisoflavone; GeniVida; Genisteol; NSC 36586; Prunetol; Sophoricol; Differenol A
More details are to be found here
Description : Genistein, a phytoestrogen found in soy products, is a highly specific inhibitor of protein tyrosine kinase (PTK) which blocks the mitogenic effect mediated by EGF on NIH-3T3 cells with IC50 of 12μM or by insulin with IC50 of 19 μM. Genistein is developed to be an antitumor agent. Genistein has antioxidant effect, it can be used in cosmetics material. - Molecular Weight :270.24
- Boiling Point :555.5±50.0°C at 760 mmHg
- Melting Point :295-300°C
- Purity :> 98%
Molecular Formula : C15H10O5 Canonical SMILES : C1=CC(=CC=C1C2=COC3=CC(=CC(=C3C2=O)O)O)O InChI : InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-13-6-10(17)5-12(18)14(13)15(11)19/h1-7,16-18H
InChIKey :
TZBJGXHYKVUXJN-UHFFFAOYSA-N
Solubility :
Soluble in DMSO (Slightly), Methanol (Slightly)
Appearance :
Pale Yellow to Light Beige Solid
Application :
antitumor agent
Storage : Store at -20°C under inert atmosphere
Synonyms :
5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; Baichanin A; Bonistein; 4',5,7-Trihydroxyisoflavone; GeniVida; Genisteol; NSC 36586; Prunetol; Sophoricol; Differenol A
More details are to be found here
Description : Genistein, a phytoestrogen found in soy products, is a highly specific inhibitor of protein tyrosine kinase (PTK) which blocks the mitogenic effect mediated by EGF on NIH-3T3 cells with IC50 of 12μM or by insulin with IC50 of 19 μM. Genistein is developed to be an antitumor agent. Genistein has antioxidant effect, it can be used in cosmetics material. - Molecular Weight :270.24
- Boiling Point :555.5±50.0°C at 760 mmHg
- Melting Point :295-300°C
- Purity :> 98%
Molecular Formula : C15H10O5 Canonical SMILES : C1=CC(=CC=C1C2=COC3=CC(=CC(=C3C2=O)O)O)O InChI : InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)11-7-20-13-6-10(17)5-12(18)14(13)15(11)19/h1-7,16-18H
InChIKey :
TZBJGXHYKVUXJN-UHFFFAOYSA-N
Solubility :
Soluble in DMSO (Slightly), Methanol (Slightly)
Appearance :
Pale Yellow to Light Beige Solid
Application :
antitumor agent
Storage : Store at -20°C under inert atmosphere
Synonyms :
5,7-Dihydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; Baichanin A; Bonistein; 4',5,7-Trihydroxyisoflavone; GeniVida; Genisteol; NSC 36586; Prunetol; Sophoricol; Differenol A
More details are to be found here
Formula: C15H10O5 Mol Weight: 270,24g/mol
More details are to be found here
Formula: C15H10O5 Mol Weight: 270,24g/mol
More details are to be found here
An isoflavone compound derived from soy products. It inhibits protein-tyrosine kinase and topoisomerase-II (DNA topoisomerase, type II) activity, and is used as an antitumor and antitumor agent. Experiments have shown that it has induced G2 phase arrest in human and mouse cell lines.
In addition, genistein has anti-worm activity. It has been identified as the active ingredient in Felmingia vestita, which is a plant traditionally used to fight worms. It has been proven to be effective in treating common liver flukes, pork flukes and poultry flukes.
In addition, genistein is a kind of phytoestrogens with selective estrogen receptor modulator properties. It has been studied in clinical trials as an alternative to classic hormone therapy to help prevent cardiovascular disease in postmenopausal women.
Natural sources of genistein include tofu, broad beans, soybeans, kudzu root and lupin.
Genistein can inhibit the growth of cancer cells by blocking enzymes required for cell growth.
Genistein can alter the transcription of cell-specific genes by interacting with nuclear estrogen receptors, thereby reducing cardiovascular risk in postmenopausal women. In randomized clinical trials, genistein can increase the ratio of nitric oxide to endothelin in healthy postmenopausal women, and improve blood flow-mediated endothelium-dependent vasodilation. 1. In addition, genistein may inhibit islet tyrosine Kinase activity and insulin release dependent on glucose and sulfonylurea drugs have beneficial effects on glucose metabolism.
An isoflavone compound derived from soy products. It inhibits protein-tyrosine kinase and topoisomerase-II (DNA topoisomerase, type II) activity, and is used as an antitumor and antitumor agent. Experiments have shown that it has induced G2 phase arrest in human and mouse cell lines.
In addition, genistein has anti-worm activity. It has been identified as the active ingredient in Felmingia vestita, which is a plant traditionally used to fight worms. It has been proven to be effective in treating common liver flukes, pork flukes and poultry flukes.
In addition, genistein is a kind of phytoestrogens with selective estrogen receptor modulator properties. It has been studied in clinical trials as an alternative to classic hormone therapy to help prevent cardiovascular disease in postmenopausal women.
Natural sources of genistein include tofu, broad beans, soybeans, kudzu root and lupin.
Genistein can inhibit the growth of cancer cells by blocking enzymes required for cell growth.
Genistein can alter the transcription of cell-specific genes by interacting with nuclear estrogen receptors, thereby reducing cardiovascular risk in postmenopausal women. In randomized clinical trials, genistein can increase the ratio of nitric oxide to endothelin in healthy postmenopausal women, and improve blood flow-mediated endothelium-dependent vasodilation. 1. In addition, genistein may inhibit islet tyrosine Kinase activity and insulin release dependent on glucose and sulfonylurea drugs have beneficial effects on glucose metabolism.
An isoflavone compound derived from soy products. It inhibits protein-tyrosine kinase and topoisomerase-II (DNA topoisomerase, type II) activity, and is used as an antitumor and antitumor agent. Experiments have shown that it has induced G2 phase arrest in human and mouse cell lines.
In addition, genistein has anti-worm activity. It has been identified as the active ingredient in Felmingia vestita, which is a plant traditionally used to fight worms. It has been proven to be effective in treating common liver flukes, pork flukes and poultry flukes.
In addition, genistein is a kind of phytoestrogens with selective estrogen receptor modulator properties. It has been studied in clinical trials as an alternative to classic hormone therapy to help prevent cardiovascular disease in postmenopausal women.
Natural sources of genistein include tofu, broad beans, soybeans, kudzu root and lupin.
Genistein can inhibit the growth of cancer cells by blocking enzymes required for cell growth.
Genistein can alter the transcription of cell-specific genes by interacting with nuclear estrogen receptors, thereby reducing cardiovascular risk in postmenopausal women. In randomized clinical trials, genistein can increase the ratio of nitric oxide to endothelin in healthy postmenopausal women, and improve blood flow-mediated endothelium-dependent vasodilation. 1. In addition, genistein may inhibit islet tyrosine Kinase activity and insulin release dependent on glucose and sulfonylurea drugs have beneficial effects on glucose metabolism.
Detailed information on the suppliers of
Genistein |
Properties:
... more properties and specification on Genistein
|
|
Privileged suppliers
Last update 2024-05-06
|


 details
details details
details details
details








