|
Suppliers for CAS
91-56-5
|
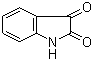
Properties | | CAS |
91-56-5 | | Formula |
C8H5NO2 | | EINECS |
202-077-8 |
|
|
25 Registered suppliers
Simagchem Corporation
P.R.China
Aecochem Corp.
P.R.China
Sancai Industry Co.,Ltd.
P.R.China
H&Z Industry Co.,Ltd
P.R.China
Xingrui Industry Co., Limited
P.R.China
Leap Chem Co., Ltd
P.R.China
Molecular Formula: C8H5NO2
Molecular Weight: 147.1308
Richman Chemical Inc.,
USA
Molecular Formula: C8H5NO2
Molecular Weight: 147.1308
Klaus F. Meyer GmbH
Germany
Molecular Formula: C8H5NO2
Molecular Weight: 147.1308
Molecular Formula: C8H5NO2
Molecular Weight: 147.1308
Krada CPS Industry S.L
Spain
Molecular Formula: C8H5NO2
Molecular Weight: 147.1308
Shandong Minglang Chemical Co., Ltd
P.R.China
Molecular Formula: C8H5NO2
Molecular Weight: 147.1308
Lori Industry Co., Ltd
P.R.China
Molecular Formula: C8H5NO2
Molecular Weight: 147.1308
Zehao Industry Co., Ltd.
P.R.China
| Appearance | Orange-red Crystal
| | Assay | 95.0%min(first) 98.0%min(medicine)
| | Moisture | 1.0%max
| | M.P. | 198-204°C |
Finetech Industry Limited
P.R.China
More details are to be found here
Hangzhou Lingrui Chemical Co., Ltd.
P.R.China
More details are to be found here
Skyrun Industrial Co., Ltd.
P.R.China
More details are to be found here
Ambeed, Inc.
USA
Purity : 98% Smile code : O=C1NC2=C(C=CC=C2)C1=O MDL Number : MFCD00005718 MolFormula : C8H5NO2 MolWeight : 147.1308 Available in stock : 134475.895
More details are to be found here
BLD Pharmatech Ltd
P.R.China
Smile code: O=C1NC2=C(C=CC=C2)C1=O MDL Number: MFCD00005718 Purity : 98% Available in stock: 87210.743 g
More details are to be found here
Xiamen Equation Chemical Co.,Ltd
P.R.China
Smile code: O=C1NC2=C(C=CC=C2)C1=O MDL Number: MFCD00005718 Purity : 98% Available in stock: 87210.743 g
More details are to be found here
AK Scientific, Inc.
USA
Smile code: O=C1NC2=C(C=CC=C2)C1=O MDL Number: MFCD00005718 Purity : 98% Available in stock: 87210.743 g
More details are to be found here
Chemos GmbH & Co. KG
Germany
Smile code: O=C1NC2=C(C=CC=C2)C1=O MDL Number: MFCD00005718 Purity : 98% Available in stock: 87210.743 g
More details are to be found here
BuGuCh & Partners
Germany
Smile code: O=C1NC2=C(C=CC=C2)C1=O MDL Number: MFCD00005718 Purity : 98% Available in stock: 87210.743 g
More details are to be found here
Product Name: Isatin
Synonyms: TIMTEC-BB SBB009100;O-AMINOPHENYLGLYOXALIC LACTIM;O-AMINOBENZOYLFORMIC ACID;O-AMINOBENZOYLFORMIC ANHYDRIDE;AKOS BBS-00003223;2,3-DIKETOINDOLINE;2,3-DIHYDROINDOLE-2,3-DIONE;2,3-INDOLEDIONE
CAS: 91-56-5
MF: C8H5NO2
MW: 147.13
EINECS: 202-077-8
mp 193-195 °C (dec.)(lit.)
Fp 220 °C
storage temp. Store at RT.
Decomposition 194 ºC
Merck 14,5104
BRN 383659
Stability: Stable. Incompatible with strong acids.
chemical property: orange-red powder or crystals
Uses
Indigo and its derivatives as indigo and related dyes and some of the raw materials for the preparation of drugs. Because of its hepatotoxicity, indigo derivatives are no longer used in the pharmaceutical industry.
A precipitant for the detection of ketones and silver. Spectrophotometric Determination of Thiophene, Thiol, Thiophene, Urine Blue and Proline.
Preparation Source
By indophenol or indigo by oxidation and prepared. Indigo was first isolated by the French chemist Auguste Laurent. In 1878, Adolf von Bayer completed the full synthesis of isatin. In 1880, Bayer developed a method of synthesizing isatin from o-nitrocinnamic acid. In 1883, from the o-nitrobenzaldehyde synthesis of isatin method by Bayer patent. Since then, indigo as the raw material for the synthesis of indigo gradually replace the method of extraction from plants, as the main source of indigo.
Fabrichem, Inc.
USA
Product Name: Isatin
Synonyms: TIMTEC-BB SBB009100;O-AMINOPHENYLGLYOXALIC LACTIM;O-AMINOBENZOYLFORMIC ACID;O-AMINOBENZOYLFORMIC ANHYDRIDE;AKOS BBS-00003223;2,3-DIKETOINDOLINE;2,3-DIHYDROINDOLE-2,3-DIONE;2,3-INDOLEDIONE
CAS: 91-56-5
MF: C8H5NO2
MW: 147.13
EINECS: 202-077-8
mp 193-195 °C (dec.)(lit.)
Fp 220 °C
storage temp. Store at RT.
Decomposition 194 ºC
Merck 14,5104
BRN 383659
Stability: Stable. Incompatible with strong acids.
chemical property: orange-red powder or crystals
Uses
Indigo and its derivatives as indigo and related dyes and some of the raw materials for the preparation of drugs. Because of its hepatotoxicity, indigo derivatives are no longer used in the pharmaceutical industry.
A precipitant for the detection of ketones and silver. Spectrophotometric Determination of Thiophene, Thiol, Thiophene, Urine Blue and Proline.
Preparation Source
By indophenol or indigo by oxidation and prepared. Indigo was first isolated by the French chemist Auguste Laurent. In 1878, Adolf von Bayer completed the full synthesis of isatin. In 1880, Bayer developed a method of synthesizing isatin from o-nitrocinnamic acid. In 1883, from the o-nitrobenzaldehyde synthesis of isatin method by Bayer patent. Since then, indigo as the raw material for the synthesis of indigo gradually replace the method of extraction from plants, as the main source of indigo.
Product Name: Isatin
Synonyms: TIMTEC-BB SBB009100;O-AMINOPHENYLGLYOXALIC LACTIM;O-AMINOBENZOYLFORMIC ACID;O-AMINOBENZOYLFORMIC ANHYDRIDE;AKOS BBS-00003223;2,3-DIKETOINDOLINE;2,3-DIHYDROINDOLE-2,3-DIONE;2,3-INDOLEDIONE
CAS: 91-56-5
MF: C8H5NO2
MW: 147.13
EINECS: 202-077-8
mp 193-195 °C (dec.)(lit.)
Fp 220 °C
storage temp. Store at RT.
Decomposition 194 ºC
Merck 14,5104
BRN 383659
Stability: Stable. Incompatible with strong acids.
chemical property: orange-red powder or crystals
Uses
Indigo and its derivatives as indigo and related dyes and some of the raw materials for the preparation of drugs. Because of its hepatotoxicity, indigo derivatives are no longer used in the pharmaceutical industry.
A precipitant for the detection of ketones and silver. Spectrophotometric Determination of Thiophene, Thiol, Thiophene, Urine Blue and Proline.
Preparation Source
By indophenol or indigo by oxidation and prepared. Indigo was first isolated by the French chemist Auguste Laurent. In 1878, Adolf von Bayer completed the full synthesis of isatin. In 1880, Bayer developed a method of synthesizing isatin from o-nitrocinnamic acid. In 1883, from the o-nitrobenzaldehyde synthesis of isatin method by Bayer patent. Since then, indigo as the raw material for the synthesis of indigo gradually replace the method of extraction from plants, as the main source of indigo.
BASF Belgium S.A. / N.V.
Belgium
Product Name: Isatin
Synonyms: TIMTEC-BB SBB009100;O-AMINOPHENYLGLYOXALIC LACTIM;O-AMINOBENZOYLFORMIC ACID;O-AMINOBENZOYLFORMIC ANHYDRIDE;AKOS BBS-00003223;2,3-DIKETOINDOLINE;2,3-DIHYDROINDOLE-2,3-DIONE;2,3-INDOLEDIONE
CAS: 91-56-5
MF: C8H5NO2
MW: 147.13
EINECS: 202-077-8
mp 193-195 °C (dec.)(lit.)
Fp 220 °C
storage temp. Store at RT.
Decomposition 194 ºC
Merck 14,5104
BRN 383659
Stability: Stable. Incompatible with strong acids.
chemical property: orange-red powder or crystals
Uses
Indigo and its derivatives as indigo and related dyes and some of the raw materials for the preparation of drugs. Because of its hepatotoxicity, indigo derivatives are no longer used in the pharmaceutical industry.
A precipitant for the detection of ketones and silver. Spectrophotometric Determination of Thiophene, Thiol, Thiophene, Urine Blue and Proline.
Preparation Source
By indophenol or indigo by oxidation and prepared. Indigo was first isolated by the French chemist Auguste Laurent. In 1878, Adolf von Bayer completed the full synthesis of isatin. In 1880, Bayer developed a method of synthesizing isatin from o-nitrocinnamic acid. In 1883, from the o-nitrobenzaldehyde synthesis of isatin method by Bayer patent. Since then, indigo as the raw material for the synthesis of indigo gradually replace the method of extraction from plants, as the main source of indigo.
Merck Schuchardt OHG
Germany
Product Name: Isatin
Synonyms: TIMTEC-BB SBB009100;O-AMINOPHENYLGLYOXALIC LACTIM;O-AMINOBENZOYLFORMIC ACID;O-AMINOBENZOYLFORMIC ANHYDRIDE;AKOS BBS-00003223;2,3-DIKETOINDOLINE;2,3-DIHYDROINDOLE-2,3-DIONE;2,3-INDOLEDIONE
CAS: 91-56-5
MF: C8H5NO2
MW: 147.13
EINECS: 202-077-8
mp 193-195 °C (dec.)(lit.)
Fp 220 °C
storage temp. Store at RT.
Decomposition 194 ºC
Merck 14,5104
BRN 383659
Stability: Stable. Incompatible with strong acids.
chemical property: orange-red powder or crystals
Uses
Indigo and its derivatives as indigo and related dyes and some of the raw materials for the preparation of drugs. Because of its hepatotoxicity, indigo derivatives are no longer used in the pharmaceutical industry.
A precipitant for the detection of ketones and silver. Spectrophotometric Determination of Thiophene, Thiol, Thiophene, Urine Blue and Proline.
Preparation Source
By indophenol or indigo by oxidation and prepared. Indigo was first isolated by the French chemist Auguste Laurent. In 1878, Adolf von Bayer completed the full synthesis of isatin. In 1880, Bayer developed a method of synthesizing isatin from o-nitrocinnamic acid. In 1883, from the o-nitrobenzaldehyde synthesis of isatin method by Bayer patent. Since then, indigo as the raw material for the synthesis of indigo gradually replace the method of extraction from plants, as the main source of indigo.
Santa Cruz Biotechnology, Inc.
USA
Product Name: Isatin
Synonyms: TIMTEC-BB SBB009100;O-AMINOPHENYLGLYOXALIC LACTIM;O-AMINOBENZOYLFORMIC ACID;O-AMINOBENZOYLFORMIC ANHYDRIDE;AKOS BBS-00003223;2,3-DIKETOINDOLINE;2,3-DIHYDROINDOLE-2,3-DIONE;2,3-INDOLEDIONE
CAS: 91-56-5
MF: C8H5NO2
MW: 147.13
EINECS: 202-077-8
mp 193-195 °C (dec.)(lit.)
Fp 220 °C
storage temp. Store at RT.
Decomposition 194 ºC
Merck 14,5104
BRN 383659
Stability: Stable. Incompatible with strong acids.
chemical property: orange-red powder or crystals
Uses
Indigo and its derivatives as indigo and related dyes and some of the raw materials for the preparation of drugs. Because of its hepatotoxicity, indigo derivatives are no longer used in the pharmaceutical industry.
A precipitant for the detection of ketones and silver. Spectrophotometric Determination of Thiophene, Thiol, Thiophene, Urine Blue and Proline.
Preparation Source
By indophenol or indigo by oxidation and prepared. Indigo was first isolated by the French chemist Auguste Laurent. In 1878, Adolf von Bayer completed the full synthesis of isatin. In 1880, Bayer developed a method of synthesizing isatin from o-nitrocinnamic acid. In 1883, from the o-nitrobenzaldehyde synthesis of isatin method by Bayer patent. Since then, indigo as the raw material for the synthesis of indigo gradually replace the method of extraction from plants, as the main source of indigo.
Detailed information on the suppliers of
Isatin |


 details
details details
details details
details








