|
Suppliers for CAS
53-16-7
|
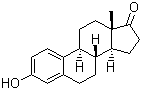
Properties | | CAS |
53-16-7 | | Formula |
C18H22O2 | | EINECS |
200-164-5 |
|
|
23 Registered suppliers
H&Z Industry Co.,Ltd
P.R.China
Xingrui Industry Co., Limited
P.R.China
Hangzhou Meite Industry Co., Ltd (Hangzhou Meite Chemical Co., Ltd)
P.R.China
Leap Chem Co., Ltd
P.R.China
Molecular Formula: C18H22O2 Molecular Weight: 270.37
More details are to be found here
Sigma-Aldrich International GmbH
Switzerland
Molecular Formula: C18H22O2 Molecular Weight: 270.37
More details are to be found here
Guangzhou Topwork Chemical Co., Ltd.
P.R.China
Molecular Formula: C18H22O2 Molecular Weight: 270.37
More details are to be found here
Wuhan PharmChem Co., LTD.
P.R.China
EINECS :200-164-5
Molecular formula :C18H22O2
X´ian Taicheng Chem Co., Ltd
P.R.China
EINECS :200-164-5
Molecular formula :C18H22O2
Shandong Ench Chemical Co.,Ltd
P.R.China
EINECS :200-164-5
Molecular formula :C18H22O2
Shandong Minglang Chemical Co., Ltd
P.R.China
EINECS :200-164-5
Molecular formula :C18H22O2
Aea.ltd
P.R.China
EINECS :200-164-5
Molecular formula :C18H22O2
Finetech Industry Limited
P.R.China
More details are to be found here
Hangzhou Lingrui Chemical Co., Ltd.
P.R.China
More details are to be found here
Skyrun Industrial Co., Ltd.
P.R.China
More details are to be found here
BOC Sciences
USA
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Shandong SanYoung Industry Co., Ltd
P.R.China
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Xiamen Equation Chemical Co.,Ltd
P.R.China
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
AK Scientific, Inc.
USA
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Chemos GmbH & Co. KG
Germany
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
BuGuCh & Partners
Germany
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Kingchem Inc.
USA
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
CM Fine Chemicals
Switzerland
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Santa Cruz Biotechnology, Inc.
USA
Description : Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver. Serum concentrations of estrone in premenopausal women fluctuate according to the menstral cycle and becomes the most predominant estrogen in postmenopausal women. The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. - Molecular Weight :270.372
- Boiling Point :445.2±45.0 °C at 760 mmHg
- Melting Point :258-260 °C
- Purity :0.95
Molecular Formula : C18H22O2 Canonical SMILES : CC12CCC3C(C1CCC2=O)CCC4=C3C=CC(=C4)O InChI : InChI=1S/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey :
DNXHEGUUPJUMQT-CBZIJGRNSA-N
Solubility :
0.03 g/L
Appearance :
White crystalline powder
Synonyms :
3-Hydroxyestra-1,3,5(10)-trien-17-one; (+)-Estrone; 1,3,5(10)-Estratrien-3-ol-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; Aquacrine; Crinovaryl; Cristallovar; Crystogen; WAY 164397; Wynestron;
More details are to be found here
Detailed information on the suppliers of
Estrone |


 details
details details
details








