|
Suppliers for CAS
81129-83-1
|
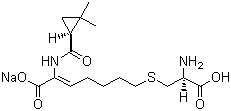
Properties | | CAS |
81129-83-1 | | Formula |
C16H25N2NaO5S | | EINECS |
279-694-4 |
|
|
14 Registered suppliers
Simagchem Corporation
P.R.China
Dayang Chem (Hangzhou) Co.,Ltd.
P.R.China
Xingrui Industry Co., Limited
P.R.China
Leap Chem Co., Ltd
P.R.China
Molecular Formula: C16H25N2O5S.Na Molecular Weight: 380.44
More details are to be found here
Shandong Minglang Chemical Co., Ltd
P.R.China
Molecular Formula: C16H25N2O5S.Na Molecular Weight: 380.44
More details are to be found here
Hangzhou Zhongqi Chem Co., Ltd
P.R.China
Molecular Formula: C16H25N2O5S.Na Molecular Weight: 380.44
More details are to be found here
Molecular Formula: C16H25N2O5S.Na Molecular Weight: 380.44
More details are to be found here
Lori Industry Co., Ltd
P.R.China
Molecular Formula: C16H25N2O5S.Na Molecular Weight: 380.44
More details are to be found here
Skyrun Industrial Co., Ltd.
P.R.China
Molecular Formula: C16H25N2O5S.Na Molecular Weight: 380.44
More details are to be found here
BOC Sciences
USA
Description : Cilastatin sodium is the sodium salt of cilastatin, which is a dipeptidase inhibitor of renal enzyme dehydropeptidase-I and leukotriene D4 peptidase. It inhibits metabolism of LTD4 to LTE4 and the hydrolysis of β-lactam antibiotics. It reduces toxic accumulation of cyclosporin A in kidney proximal tubule epithelial cells. It can be combined intravenously with imipenem in order to protect it from dehydropeptidase. It suppresses both host and target metabolism of the broad-spectrum antibiotic imipenem, improving its efficacy. It usually confers antibiotic resistance to certain bacteria because itself does not have antibiotic activity. It has nephroprotective effects. - Molecular Weight :380.43
- Purity :≥99% by HPLC
Molecular Formula : C16H25N2NaO5S Canonical SMILES : CC1(CC1C(=O)NC(=CCCCCSCC(C(=O)O)N)C(=O)[O-])C.[Na+]
InChI :
InChI=1S/C16H26N2O5S.Na/c1-16(2)8-10(16)13(19)18-12(15(22)23)6-4-3-5-7-24-9-11(17)14(20)21;/h6,10-11H,3-5,7-9,17H2,1-2H3,(H,18,19)(H,20,21)(H,22,23);/q;+1/p-1/b12-6-;/t10-,11+;/m1./s1
InChIKey :
QXPBTTUOVWMPJN-QBNHLFMHSA-M
Synonyms :
L 642957; MK 791; L642957; MK791; L-642957; MK-791; [R-[R*,S*-(Z)]]-7-[(2-Amino-2-carboxyethyl)thio]-2-[[(2,2-dimethylcyclopropyl)carbonyl]amino]-2-heptenoic Acid Monosodium Salt; (2Z)-7-[[(2R)-2-Amino-2-carboxyethyl]thio]-2-[[[(1S)-2,2-dimethylcyclopropyl]carbonyl]amino]-2-heptenoic Acid Sodium Salt; sodium S-((Z)-6-carboxy-6-((S)-2,2-dimethylcyclopropane-1-carboxamido)hex-5-en-1-yl)-L-cysteinate
More details are to be found here
Xiamen Equation Chemical Co.,Ltd
P.R.China
Description : Cilastatin sodium is the sodium salt of cilastatin, which is a dipeptidase inhibitor of renal enzyme dehydropeptidase-I and leukotriene D4 peptidase. It inhibits metabolism of LTD4 to LTE4 and the hydrolysis of β-lactam antibiotics. It reduces toxic accumulation of cyclosporin A in kidney proximal tubule epithelial cells. It can be combined intravenously with imipenem in order to protect it from dehydropeptidase. It suppresses both host and target metabolism of the broad-spectrum antibiotic imipenem, improving its efficacy. It usually confers antibiotic resistance to certain bacteria because itself does not have antibiotic activity. It has nephroprotective effects. - Molecular Weight :380.43
- Purity :≥99% by HPLC
Molecular Formula : C16H25N2NaO5S Canonical SMILES : CC1(CC1C(=O)NC(=CCCCCSCC(C(=O)O)N)C(=O)[O-])C.[Na+]
InChI :
InChI=1S/C16H26N2O5S.Na/c1-16(2)8-10(16)13(19)18-12(15(22)23)6-4-3-5-7-24-9-11(17)14(20)21;/h6,10-11H,3-5,7-9,17H2,1-2H3,(H,18,19)(H,20,21)(H,22,23);/q;+1/p-1/b12-6-;/t10-,11+;/m1./s1
InChIKey :
QXPBTTUOVWMPJN-QBNHLFMHSA-M
Synonyms :
L 642957; MK 791; L642957; MK791; L-642957; MK-791; [R-[R*,S*-(Z)]]-7-[(2-Amino-2-carboxyethyl)thio]-2-[[(2,2-dimethylcyclopropyl)carbonyl]amino]-2-heptenoic Acid Monosodium Salt; (2Z)-7-[[(2R)-2-Amino-2-carboxyethyl]thio]-2-[[[(1S)-2,2-dimethylcyclopropyl]carbonyl]amino]-2-heptenoic Acid Sodium Salt; sodium S-((Z)-6-carboxy-6-((S)-2,2-dimethylcyclopropane-1-carboxamido)hex-5-en-1-yl)-L-cysteinate
More details are to be found here
AK Scientific, Inc.
USA
Description : Cilastatin sodium is the sodium salt of cilastatin, which is a dipeptidase inhibitor of renal enzyme dehydropeptidase-I and leukotriene D4 peptidase. It inhibits metabolism of LTD4 to LTE4 and the hydrolysis of β-lactam antibiotics. It reduces toxic accumulation of cyclosporin A in kidney proximal tubule epithelial cells. It can be combined intravenously with imipenem in order to protect it from dehydropeptidase. It suppresses both host and target metabolism of the broad-spectrum antibiotic imipenem, improving its efficacy. It usually confers antibiotic resistance to certain bacteria because itself does not have antibiotic activity. It has nephroprotective effects. - Molecular Weight :380.43
- Purity :≥99% by HPLC
Molecular Formula : C16H25N2NaO5S Canonical SMILES : CC1(CC1C(=O)NC(=CCCCCSCC(C(=O)O)N)C(=O)[O-])C.[Na+]
InChI :
InChI=1S/C16H26N2O5S.Na/c1-16(2)8-10(16)13(19)18-12(15(22)23)6-4-3-5-7-24-9-11(17)14(20)21;/h6,10-11H,3-5,7-9,17H2,1-2H3,(H,18,19)(H,20,21)(H,22,23);/q;+1/p-1/b12-6-;/t10-,11+;/m1./s1
InChIKey :
QXPBTTUOVWMPJN-QBNHLFMHSA-M
Synonyms :
L 642957; MK 791; L642957; MK791; L-642957; MK-791; [R-[R*,S*-(Z)]]-7-[(2-Amino-2-carboxyethyl)thio]-2-[[(2,2-dimethylcyclopropyl)carbonyl]amino]-2-heptenoic Acid Monosodium Salt; (2Z)-7-[[(2R)-2-Amino-2-carboxyethyl]thio]-2-[[[(1S)-2,2-dimethylcyclopropyl]carbonyl]amino]-2-heptenoic Acid Sodium Salt; sodium S-((Z)-6-carboxy-6-((S)-2,2-dimethylcyclopropane-1-carboxamido)hex-5-en-1-yl)-L-cysteinate
More details are to be found here
Chemos GmbH & Co. KG
Germany
Description : Cilastatin sodium is the sodium salt of cilastatin, which is a dipeptidase inhibitor of renal enzyme dehydropeptidase-I and leukotriene D4 peptidase. It inhibits metabolism of LTD4 to LTE4 and the hydrolysis of β-lactam antibiotics. It reduces toxic accumulation of cyclosporin A in kidney proximal tubule epithelial cells. It can be combined intravenously with imipenem in order to protect it from dehydropeptidase. It suppresses both host and target metabolism of the broad-spectrum antibiotic imipenem, improving its efficacy. It usually confers antibiotic resistance to certain bacteria because itself does not have antibiotic activity. It has nephroprotective effects. - Molecular Weight :380.43
- Purity :≥99% by HPLC
Molecular Formula : C16H25N2NaO5S Canonical SMILES : CC1(CC1C(=O)NC(=CCCCCSCC(C(=O)O)N)C(=O)[O-])C.[Na+]
InChI :
InChI=1S/C16H26N2O5S.Na/c1-16(2)8-10(16)13(19)18-12(15(22)23)6-4-3-5-7-24-9-11(17)14(20)21;/h6,10-11H,3-5,7-9,17H2,1-2H3,(H,18,19)(H,20,21)(H,22,23);/q;+1/p-1/b12-6-;/t10-,11+;/m1./s1
InChIKey :
QXPBTTUOVWMPJN-QBNHLFMHSA-M
Synonyms :
L 642957; MK 791; L642957; MK791; L-642957; MK-791; [R-[R*,S*-(Z)]]-7-[(2-Amino-2-carboxyethyl)thio]-2-[[(2,2-dimethylcyclopropyl)carbonyl]amino]-2-heptenoic Acid Monosodium Salt; (2Z)-7-[[(2R)-2-Amino-2-carboxyethyl]thio]-2-[[[(1S)-2,2-dimethylcyclopropyl]carbonyl]amino]-2-heptenoic Acid Sodium Salt; sodium S-((Z)-6-carboxy-6-((S)-2,2-dimethylcyclopropane-1-carboxamido)hex-5-en-1-yl)-L-cysteinate
More details are to be found here
BuGuCh & Partners
Germany
Description : Cilastatin sodium is the sodium salt of cilastatin, which is a dipeptidase inhibitor of renal enzyme dehydropeptidase-I and leukotriene D4 peptidase. It inhibits metabolism of LTD4 to LTE4 and the hydrolysis of β-lactam antibiotics. It reduces toxic accumulation of cyclosporin A in kidney proximal tubule epithelial cells. It can be combined intravenously with imipenem in order to protect it from dehydropeptidase. It suppresses both host and target metabolism of the broad-spectrum antibiotic imipenem, improving its efficacy. It usually confers antibiotic resistance to certain bacteria because itself does not have antibiotic activity. It has nephroprotective effects. - Molecular Weight :380.43
- Purity :≥99% by HPLC
Molecular Formula : C16H25N2NaO5S Canonical SMILES : CC1(CC1C(=O)NC(=CCCCCSCC(C(=O)O)N)C(=O)[O-])C.[Na+]
InChI :
InChI=1S/C16H26N2O5S.Na/c1-16(2)8-10(16)13(19)18-12(15(22)23)6-4-3-5-7-24-9-11(17)14(20)21;/h6,10-11H,3-5,7-9,17H2,1-2H3,(H,18,19)(H,20,21)(H,22,23);/q;+1/p-1/b12-6-;/t10-,11+;/m1./s1
InChIKey :
QXPBTTUOVWMPJN-QBNHLFMHSA-M
Synonyms :
L 642957; MK 791; L642957; MK791; L-642957; MK-791; [R-[R*,S*-(Z)]]-7-[(2-Amino-2-carboxyethyl)thio]-2-[[(2,2-dimethylcyclopropyl)carbonyl]amino]-2-heptenoic Acid Monosodium Salt; (2Z)-7-[[(2R)-2-Amino-2-carboxyethyl]thio]-2-[[[(1S)-2,2-dimethylcyclopropyl]carbonyl]amino]-2-heptenoic Acid Sodium Salt; sodium S-((Z)-6-carboxy-6-((S)-2,2-dimethylcyclopropane-1-carboxamido)hex-5-en-1-yl)-L-cysteinate
More details are to be found here
Santa Cruz Biotechnology, Inc.
USA
Description : Cilastatin sodium is the sodium salt of cilastatin, which is a dipeptidase inhibitor of renal enzyme dehydropeptidase-I and leukotriene D4 peptidase. It inhibits metabolism of LTD4 to LTE4 and the hydrolysis of β-lactam antibiotics. It reduces toxic accumulation of cyclosporin A in kidney proximal tubule epithelial cells. It can be combined intravenously with imipenem in order to protect it from dehydropeptidase. It suppresses both host and target metabolism of the broad-spectrum antibiotic imipenem, improving its efficacy. It usually confers antibiotic resistance to certain bacteria because itself does not have antibiotic activity. It has nephroprotective effects. - Molecular Weight :380.43
- Purity :≥99% by HPLC
Molecular Formula : C16H25N2NaO5S Canonical SMILES : CC1(CC1C(=O)NC(=CCCCCSCC(C(=O)O)N)C(=O)[O-])C.[Na+]
InChI :
InChI=1S/C16H26N2O5S.Na/c1-16(2)8-10(16)13(19)18-12(15(22)23)6-4-3-5-7-24-9-11(17)14(20)21;/h6,10-11H,3-5,7-9,17H2,1-2H3,(H,18,19)(H,20,21)(H,22,23);/q;+1/p-1/b12-6-;/t10-,11+;/m1./s1
InChIKey :
QXPBTTUOVWMPJN-QBNHLFMHSA-M
Synonyms :
L 642957; MK 791; L642957; MK791; L-642957; MK-791; [R-[R*,S*-(Z)]]-7-[(2-Amino-2-carboxyethyl)thio]-2-[[(2,2-dimethylcyclopropyl)carbonyl]amino]-2-heptenoic Acid Monosodium Salt; (2Z)-7-[[(2R)-2-Amino-2-carboxyethyl]thio]-2-[[[(1S)-2,2-dimethylcyclopropyl]carbonyl]amino]-2-heptenoic Acid Sodium Salt; sodium S-((Z)-6-carboxy-6-((S)-2,2-dimethylcyclopropane-1-carboxamido)hex-5-en-1-yl)-L-cysteinate
More details are to be found here
|


 details
details details
details
_Co_Ltd_1_575.jpg)







