|
|
|

|
Suppliers for
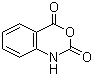
Isatoic anhydride
|
Properties | | CAS |
118-48-9 | | Formula |
C8H5NO3 | | EINECS |
204-255-0 |
|
|
27 Registered suppliers
| Appearance | Light brown powder
| | HPLC purity | 99.0% Min
| | Moisture | 1.0% Max
| | Ash | 0.5% Max |
| Appearance | Light brown powder
| | HPLC purity | 99.0% Min
| | Moisture | 1.0% Max
| | Ash | 0.5% Max |
| Appearance | Light brown powder
| | HPLC purity | 99.0% Min
| | Moisture | 1.0% Max
| | Ash | 0.5% Max |
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Molecular Formula: C8H5NO3
Molecular Weight: 163.13
Hazard Symbols: Xi
WGKGermany: 1
HS Code: 29349990
Product name: ISATOIC ANHYDRIDE
Synonyms:1,2-Dihydro-3,1-benzoxazine-2,4-dione;2-(carboxyamino)-benzoicacicyclicanhydride;2,4-Dioxo-1,2-dihydro-4H-3,1-benzoxazine;4H-3,1-Benzoxazin-2,4-(1H)-dion;4H-3,1-Benzoxazin-2,4(1H)-dion;Anthranilic acid, N-carboxy-, cyclic anhydride;Benzoic acid, 2-(carboxyamino)-, cyclic anhydride;Benzoicacid,2-(carboxyamino)-,cyclicanhydride
CAS: 118-48-9
Formula: C8H5NO3
Molecular weight: 163.13
Chemical Properties:White prismatic crystals. Melting point 243 ℃ (decomposition). Soluble in ethanol, 1,4-dioxane.
Purity : 98%min
Water : 0.5%max
Melting point: 233 °C (dec.)(lit.)
Density: 1,52 g/cm3
Vapor Density: 5.6 (vs air)
Flesh point: 308 °C
Water solubility: decomposes
Sensitivity: Moisture Sensitive
BRN: 136786
Usage:
1.Starch anthranilate (Starch anthranilate) in the paper industry can improve the inorganic filler and fines retention, but also can improve the dry strength and tensile strength.
A variety of solvents and in the presence of various catalysts, starch anthranilate was obtained by the reaction of isatoic anhydride with starch system. The method is based on classical triethylamine as a catalyst, the reaction in dimethyl sulfoxide solvent.
(1) 40g of starch (dried overnight at 100 deg.] C), 4.0 g of isatoic anhydride, 5ml of triethylamine in 500ml dimethyl sulfoxide, 90 ~ 95 ℃ reaction was stirred 3h, at room temperature overnight, the The resulting light brown liquid was poured into 1L of methanol to precipitate washed with methanol, dried to obtain a white powder sample. Nitrogen 0.75%, the degree of substitution of 0.093.
(2)in an aqueous solution. 90g of cornstarch is dispersed in 250ml of water, 8g isatoic anhydride was added over 30 minutes, with 6% sodium hydroxide solution and adjusted to pH 8.5 to 9.0, maintaining the pH in the reaction time 2 hours. After the reaction was filtered, washed with 2L, dry place Soxhlet extraction, continuous extraction with ethanol 24h. The resulting product containing 0.70% nitrogen, available gum base degree of substitution (DS) of 0.086.
2.As a raw material medicine, pesticides, dyes, and it is an important intermediate herbicide bentazon
Brief manufactured process:
Anthranilic acid and phosgene reactions derived. Anthranilic acid was dissolved in hot water and concentrated hydrochloric acid under stirring phosgene, isatoic anhydride immediately precipitated. Exothermic reaction, adjust the speed of light gas into the temperature does not exceed 50 ℃. Continuous phosgene 2-4h, to significantly slow down the absorption rate so far. The product was dried in air at 100 ℃ drying derived products.
Product name: ISATOIC ANHYDRIDE
Synonyms:1,2-Dihydro-3,1-benzoxazine-2,4-dione;2-(carboxyamino)-benzoicacicyclicanhydride;2,4-Dioxo-1,2-dihydro-4H-3,1-benzoxazine;4H-3,1-Benzoxazin-2,4-(1H)-dion;4H-3,1-Benzoxazin-2,4(1H)-dion;Anthranilic acid, N-carboxy-, cyclic anhydride;Benzoic acid, 2-(carboxyamino)-, cyclic anhydride;Benzoicacid,2-(carboxyamino)-,cyclicanhydride
CAS: 118-48-9
Formula: C8H5NO3
Molecular weight: 163.13
Chemical Properties:White prismatic crystals. Melting point 243 ℃ (decomposition). Soluble in ethanol, 1,4-dioxane.
Purity : 98%min
Water : 0.5%max
Melting point: 233 °C (dec.)(lit.)
Density: 1,52 g/cm3
Vapor Density: 5.6 (vs air)
Flesh point: 308 °C
Water solubility: decomposes
Sensitivity: Moisture Sensitive
BRN: 136786
Usage:
1.Starch anthranilate (Starch anthranilate) in the paper industry can improve the inorganic filler and fines retention, but also can improve the dry strength and tensile strength.
A variety of solvents and in the presence of various catalysts, starch anthranilate was obtained by the reaction of isatoic anhydride with starch system. The method is based on classical triethylamine as a catalyst, the reaction in dimethyl sulfoxide solvent.
(1) 40g of starch (dried overnight at 100 deg.] C), 4.0 g of isatoic anhydride, 5ml of triethylamine in 500ml dimethyl sulfoxide, 90 ~ 95 ℃ reaction was stirred 3h, at room temperature overnight, the The resulting light brown liquid was poured into 1L of methanol to precipitate washed with methanol, dried to obtain a white powder sample. Nitrogen 0.75%, the degree of substitution of 0.093.
(2)in an aqueous solution. 90g of cornstarch is dispersed in 250ml of water, 8g isatoic anhydride was added over 30 minutes, with 6% sodium hydroxide solution and adjusted to pH 8.5 to 9.0, maintaining the pH in the reaction time 2 hours. After the reaction was filtered, washed with 2L, dry place Soxhlet extraction, continuous extraction with ethanol 24h. The resulting product containing 0.70% nitrogen, available gum base degree of substitution (DS) of 0.086.
2.As a raw material medicine, pesticides, dyes, and it is an important intermediate herbicide bentazon
Brief manufactured process:
Anthranilic acid and phosgene reactions derived. Anthranilic acid was dissolved in hot water and concentrated hydrochloric acid under stirring phosgene, isatoic anhydride immediately precipitated. Exothermic reaction, adjust the speed of light gas into the temperature does not exceed 50 ℃. Continuous phosgene 2-4h, to significantly slow down the absorption rate so far. The product was dried in air at 100 ℃ drying derived products.
Product name: ISATOIC ANHYDRIDE
Synonyms:1,2-Dihydro-3,1-benzoxazine-2,4-dione;2-(carboxyamino)-benzoicacicyclicanhydride;2,4-Dioxo-1,2-dihydro-4H-3,1-benzoxazine;4H-3,1-Benzoxazin-2,4-(1H)-dion;4H-3,1-Benzoxazin-2,4(1H)-dion;Anthranilic acid, N-carboxy-, cyclic anhydride;Benzoic acid, 2-(carboxyamino)-, cyclic anhydride;Benzoicacid,2-(carboxyamino)-,cyclicanhydride
CAS: 118-48-9
Formula: C8H5NO3
Molecular weight: 163.13
Chemical Properties:White prismatic crystals. Melting point 243 ℃ (decomposition). Soluble in ethanol, 1,4-dioxane.
Purity : 98%min
Water : 0.5%max
Melting point: 233 °C (dec.)(lit.)
Density: 1,52 g/cm3
Vapor Density: 5.6 (vs air)
Flesh point: 308 °C
Water solubility: decomposes
Sensitivity: Moisture Sensitive
BRN: 136786
Usage:
1.Starch anthranilate (Starch anthranilate) in the paper industry can improve the inorganic filler and fines retention, but also can improve the dry strength and tensile strength.
A variety of solvents and in the presence of various catalysts, starch anthranilate was obtained by the reaction of isatoic anhydride with starch system. The method is based on classical triethylamine as a catalyst, the reaction in dimethyl sulfoxide solvent.
(1) 40g of starch (dried overnight at 100 deg.] C), 4.0 g of isatoic anhydride, 5ml of triethylamine in 500ml dimethyl sulfoxide, 90 ~ 95 ℃ reaction was stirred 3h, at room temperature overnight, the The resulting light brown liquid was poured into 1L of methanol to precipitate washed with methanol, dried to obtain a white powder sample. Nitrogen 0.75%, the degree of substitution of 0.093.
(2)in an aqueous solution. 90g of cornstarch is dispersed in 250ml of water, 8g isatoic anhydride was added over 30 minutes, with 6% sodium hydroxide solution and adjusted to pH 8.5 to 9.0, maintaining the pH in the reaction time 2 hours. After the reaction was filtered, washed with 2L, dry place Soxhlet extraction, continuous extraction with ethanol 24h. The resulting product containing 0.70% nitrogen, available gum base degree of substitution (DS) of 0.086.
2.As a raw material medicine, pesticides, dyes, and it is an important intermediate herbicide bentazon
Brief manufactured process:
Anthranilic acid and phosgene reactions derived. Anthranilic acid was dissolved in hot water and concentrated hydrochloric acid under stirring phosgene, isatoic anhydride immediately precipitated. Exothermic reaction, adjust the speed of light gas into the temperature does not exceed 50 ℃. Continuous phosgene 2-4h, to significantly slow down the absorption rate so far. The product was dried in air at 100 ℃ drying derived products.
Product name: ISATOIC ANHYDRIDE
Synonyms:1,2-Dihydro-3,1-benzoxazine-2,4-dione;2-(carboxyamino)-benzoicacicyclicanhydride;2,4-Dioxo-1,2-dihydro-4H-3,1-benzoxazine;4H-3,1-Benzoxazin-2,4-(1H)-dion;4H-3,1-Benzoxazin-2,4(1H)-dion;Anthranilic acid, N-carboxy-, cyclic anhydride;Benzoic acid, 2-(carboxyamino)-, cyclic anhydride;Benzoicacid,2-(carboxyamino)-,cyclicanhydride
CAS: 118-48-9
Formula: C8H5NO3
Molecular weight: 163.13
Chemical Properties:White prismatic crystals. Melting point 243 ℃ (decomposition). Soluble in ethanol, 1,4-dioxane.
Purity : 98%min
Water : 0.5%max
Melting point: 233 °C (dec.)(lit.)
Density: 1,52 g/cm3
Vapor Density: 5.6 (vs air)
Flesh point: 308 °C
Water solubility: decomposes
Sensitivity: Moisture Sensitive
BRN: 136786
Usage:
1.Starch anthranilate (Starch anthranilate) in the paper industry can improve the inorganic filler and fines retention, but also can improve the dry strength and tensile strength.
A variety of solvents and in the presence of various catalysts, starch anthranilate was obtained by the reaction of isatoic anhydride with starch system. The method is based on classical triethylamine as a catalyst, the reaction in dimethyl sulfoxide solvent.
(1) 40g of starch (dried overnight at 100 deg.] C), 4.0 g of isatoic anhydride, 5ml of triethylamine in 500ml dimethyl sulfoxide, 90 ~ 95 ℃ reaction was stirred 3h, at room temperature overnight, the The resulting light brown liquid was poured into 1L of methanol to precipitate washed with methanol, dried to obtain a white powder sample. Nitrogen 0.75%, the degree of substitution of 0.093.
(2)in an aqueous solution. 90g of cornstarch is dispersed in 250ml of water, 8g isatoic anhydride was added over 30 minutes, with 6% sodium hydroxide solution and adjusted to pH 8.5 to 9.0, maintaining the pH in the reaction time 2 hours. After the reaction was filtered, washed with 2L, dry place Soxhlet extraction, continuous extraction with ethanol 24h. The resulting product containing 0.70% nitrogen, available gum base degree of substitution (DS) of 0.086.
2.As a raw material medicine, pesticides, dyes, and it is an important intermediate herbicide bentazon
Brief manufactured process:
Anthranilic acid and phosgene reactions derived. Anthranilic acid was dissolved in hot water and concentrated hydrochloric acid under stirring phosgene, isatoic anhydride immediately precipitated. Exothermic reaction, adjust the speed of light gas into the temperature does not exceed 50 ℃. Continuous phosgene 2-4h, to significantly slow down the absorption rate so far. The product was dried in air at 100 ℃ drying derived products.
Product name: ISATOIC ANHYDRIDE
Synonyms:1,2-Dihydro-3,1-benzoxazine-2,4-dione;2-(carboxyamino)-benzoicacicyclicanhydride;2,4-Dioxo-1,2-dihydro-4H-3,1-benzoxazine;4H-3,1-Benzoxazin-2,4-(1H)-dion;4H-3,1-Benzoxazin-2,4(1H)-dion;Anthranilic acid, N-carboxy-, cyclic anhydride;Benzoic acid, 2-(carboxyamino)-, cyclic anhydride;Benzoicacid,2-(carboxyamino)-,cyclicanhydride
CAS: 118-48-9
Formula: C8H5NO3
Molecular weight: 163.13
Chemical Properties:White prismatic crystals. Melting point 243 ℃ (decomposition). Soluble in ethanol, 1,4-dioxane.
Purity : 98%min
Water : 0.5%max
Melting point: 233 °C (dec.)(lit.)
Density: 1,52 g/cm3
Vapor Density: 5.6 (vs air)
Flesh point: 308 °C
Water solubility: decomposes
Sensitivity: Moisture Sensitive
BRN: 136786
Usage:
1.Starch anthranilate (Starch anthranilate) in the paper industry can improve the inorganic filler and fines retention, but also can improve the dry strength and tensile strength.
A variety of solvents and in the presence of various catalysts, starch anthranilate was obtained by the reaction of isatoic anhydride with starch system. The method is based on classical triethylamine as a catalyst, the reaction in dimethyl sulfoxide solvent.
(1) 40g of starch (dried overnight at 100 deg.] C), 4.0 g of isatoic anhydride, 5ml of triethylamine in 500ml dimethyl sulfoxide, 90 ~ 95 ℃ reaction was stirred 3h, at room temperature overnight, the The resulting light brown liquid was poured into 1L of methanol to precipitate washed with methanol, dried to obtain a white powder sample. Nitrogen 0.75%, the degree of substitution of 0.093.
(2)in an aqueous solution. 90g of cornstarch is dispersed in 250ml of water, 8g isatoic anhydride was added over 30 minutes, with 6% sodium hydroxide solution and adjusted to pH 8.5 to 9.0, maintaining the pH in the reaction time 2 hours. After the reaction was filtered, washed with 2L, dry place Soxhlet extraction, continuous extraction with ethanol 24h. The resulting product containing 0.70% nitrogen, available gum base degree of substitution (DS) of 0.086.
2.As a raw material medicine, pesticides, dyes, and it is an important intermediate herbicide bentazon
Brief manufactured process:
Anthranilic acid and phosgene reactions derived. Anthranilic acid was dissolved in hot water and concentrated hydrochloric acid under stirring phosgene, isatoic anhydride immediately precipitated. Exothermic reaction, adjust the speed of light gas into the temperature does not exceed 50 ℃. Continuous phosgene 2-4h, to significantly slow down the absorption rate so far. The product was dried in air at 100 ℃ drying derived products.
Product name: ISATOIC ANHYDRIDE
Synonyms:1,2-Dihydro-3,1-benzoxazine-2,4-dione;2-(carboxyamino)-benzoicacicyclicanhydride;2,4-Dioxo-1,2-dihydro-4H-3,1-benzoxazine;4H-3,1-Benzoxazin-2,4-(1H)-dion;4H-3,1-Benzoxazin-2,4(1H)-dion;Anthranilic acid, N-carboxy-, cyclic anhydride;Benzoic acid, 2-(carboxyamino)-, cyclic anhydride;Benzoicacid,2-(carboxyamino)-,cyclicanhydride
CAS: 118-48-9
Formula: C8H5NO3
Molecular weight: 163.13
Chemical Properties:White prismatic crystals. Melting point 243 ℃ (decomposition). Soluble in ethanol, 1,4-dioxane.
Purity : 98%min
Water : 0.5%max
Melting point: 233 °C (dec.)(lit.)
Density: 1,52 g/cm3
Vapor Density: 5.6 (vs air)
Flesh point: 308 °C
Water solubility: decomposes
Sensitivity: Moisture Sensitive
BRN: 136786
Usage:
1.Starch anthranilate (Starch anthranilate) in the paper industry can improve the inorganic filler and fines retention, but also can improve the dry strength and tensile strength.
A variety of solvents and in the presence of various catalysts, starch anthranilate was obtained by the reaction of isatoic anhydride with starch system. The method is based on classical triethylamine as a catalyst, the reaction in dimethyl sulfoxide solvent.
(1) 40g of starch (dried overnight at 100 deg.] C), 4.0 g of isatoic anhydride, 5ml of triethylamine in 500ml dimethyl sulfoxide, 90 ~ 95 ℃ reaction was stirred 3h, at room temperature overnight, the The resulting light brown liquid was poured into 1L of methanol to precipitate washed with methanol, dried to obtain a white powder sample. Nitrogen 0.75%, the degree of substitution of 0.093.
(2)in an aqueous solution. 90g of cornstarch is dispersed in 250ml of water, 8g isatoic anhydride was added over 30 minutes, with 6% sodium hydroxide solution and adjusted to pH 8.5 to 9.0, maintaining the pH in the reaction time 2 hours. After the reaction was filtered, washed with 2L, dry place Soxhlet extraction, continuous extraction with ethanol 24h. The resulting product containing 0.70% nitrogen, available gum base degree of substitution (DS) of 0.086.
2.As a raw material medicine, pesticides, dyes, and it is an important intermediate herbicide bentazon
Brief manufactured process:
Anthranilic acid and phosgene reactions derived. Anthranilic acid was dissolved in hot water and concentrated hydrochloric acid under stirring phosgene, isatoic anhydride immediately precipitated. Exothermic reaction, adjust the speed of light gas into the temperature does not exceed 50 ℃. Continuous phosgene 2-4h, to significantly slow down the absorption rate so far. The product was dried in air at 100 ℃ drying derived products.
Product name: ISATOIC ANHYDRIDE
Synonyms:1,2-Dihydro-3,1-benzoxazine-2,4-dione;2-(carboxyamino)-benzoicacicyclicanhydride;2,4-Dioxo-1,2-dihydro-4H-3,1-benzoxazine;4H-3,1-Benzoxazin-2,4-(1H)-dion;4H-3,1-Benzoxazin-2,4(1H)-dion;Anthranilic acid, N-carboxy-, cyclic anhydride;Benzoic acid, 2-(carboxyamino)-, cyclic anhydride;Benzoicacid,2-(carboxyamino)-,cyclicanhydride
CAS: 118-48-9
Formula: C8H5NO3
Molecular weight: 163.13
Chemical Properties:White prismatic crystals. Melting point 243 ℃ (decomposition). Soluble in ethanol, 1,4-dioxane.
Purity : 98%min
Water : 0.5%max
Melting point: 233 °C (dec.)(lit.)
Density: 1,52 g/cm3
Vapor Density: 5.6 (vs air)
Flesh point: 308 °C
Water solubility: decomposes
Sensitivity: Moisture Sensitive
BRN: 136786
Usage:
1.Starch anthranilate (Starch anthranilate) in the paper industry can improve the inorganic filler and fines retention, but also can improve the dry strength and tensile strength.
A variety of solvents and in the presence of various catalysts, starch anthranilate was obtained by the reaction of isatoic anhydride with starch system. The method is based on classical triethylamine as a catalyst, the reaction in dimethyl sulfoxide solvent.
(1) 40g of starch (dried overnight at 100 deg.] C), 4.0 g of isatoic anhydride, 5ml of triethylamine in 500ml dimethyl sulfoxide, 90 ~ 95 ℃ reaction was stirred 3h, at room temperature overnight, the The resulting light brown liquid was poured into 1L of methanol to precipitate washed with methanol, dried to obtain a white powder sample. Nitrogen 0.75%, the degree of substitution of 0.093.
(2)in an aqueous solution. 90g of cornstarch is dispersed in 250ml of water, 8g isatoic anhydride was added over 30 minutes, with 6% sodium hydroxide solution and adjusted to pH 8.5 to 9.0, maintaining the pH in the reaction time 2 hours. After the reaction was filtered, washed with 2L, dry place Soxhlet extraction, continuous extraction with ethanol 24h. The resulting product containing 0.70% nitrogen, available gum base degree of substitution (DS) of 0.086.
2.As a raw material medicine, pesticides, dyes, and it is an important intermediate herbicide bentazon
Brief manufactured process:
Anthranilic acid and phosgene reactions derived. Anthranilic acid was dissolved in hot water and concentrated hydrochloric acid under stirring phosgene, isatoic anhydride immediately precipitated. Exothermic reaction, adjust the speed of light gas into the temperature does not exceed 50 ℃. Continuous phosgene 2-4h, to significantly slow down the absorption rate so far. The product was dried in air at 100 ℃ drying derived products.
Product name: ISATOIC ANHYDRIDE
Synonyms:1,2-Dihydro-3,1-benzoxazine-2,4-dione;2-(carboxyamino)-benzoicacicyclicanhydride;2,4-Dioxo-1,2-dihydro-4H-3,1-benzoxazine;4H-3,1-Benzoxazin-2,4-(1H)-dion;4H-3,1-Benzoxazin-2,4(1H)-dion;Anthranilic acid, N-carboxy-, cyclic anhydride;Benzoic acid, 2-(carboxyamino)-, cyclic anhydride;Benzoicacid,2-(carboxyamino)-,cyclicanhydride
CAS: 118-48-9
Formula: C8H5NO3
Molecular weight: 163.13
Chemical Properties:White prismatic crystals. Melting point 243 ℃ (decomposition). Soluble in ethanol, 1,4-dioxane.
Purity : 98%min
Water : 0.5%max
Melting point: 233 °C (dec.)(lit.)
Density: 1,52 g/cm3
Vapor Density: 5.6 (vs air)
Flesh point: 308 °C
Water solubility: decomposes
Sensitivity: Moisture Sensitive
BRN: 136786
Usage:
1.Starch anthranilate (Starch anthranilate) in the paper industry can improve the inorganic filler and fines retention, but also can improve the dry strength and tensile strength.
A variety of solvents and in the presence of various catalysts, starch anthranilate was obtained by the reaction of isatoic anhydride with starch system. The method is based on classical triethylamine as a catalyst, the reaction in dimethyl sulfoxide solvent.
(1) 40g of starch (dried overnight at 100 deg.] C), 4.0 g of isatoic anhydride, 5ml of triethylamine in 500ml dimethyl sulfoxide, 90 ~ 95 ℃ reaction was stirred 3h, at room temperature overnight, the The resulting light brown liquid was poured into 1L of methanol to precipitate washed with methanol, dried to obtain a white powder sample. Nitrogen 0.75%, the degree of substitution of 0.093.
(2)in an aqueous solution. 90g of cornstarch is dispersed in 250ml of water, 8g isatoic anhydride was added over 30 minutes, with 6% sodium hydroxide solution and adjusted to pH 8.5 to 9.0, maintaining the pH in the reaction time 2 hours. After the reaction was filtered, washed with 2L, dry place Soxhlet extraction, continuous extraction with ethanol 24h. The resulting product containing 0.70% nitrogen, available gum base degree of substitution (DS) of 0.086.
2.As a raw material medicine, pesticides, dyes, and it is an important intermediate herbicide bentazon
Brief manufactured process:
Anthranilic acid and phosgene reactions derived. Anthranilic acid was dissolved in hot water and concentrated hydrochloric acid under stirring phosgene, isatoic anhydride immediately precipitated. Exothermic reaction, adjust the speed of light gas into the temperature does not exceed 50 ℃. Continuous phosgene 2-4h, to significantly slow down the absorption rate so far. The product was dried in air at 100 ℃ drying derived products.
|
|
|
Privileged suppliers
Last update 2024-04-26
|


 details
details details
details








