|
Suppliers for CAS
1303-86-2
|
Properties | | CAS |
1303-86-2 | | Formula |
B2O3 | | EINECS |
215-125-8 |
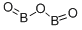
|
|
23 Registered suppliers
Simagchem Corporation
P.R.China
Aecochem Corp.
P.R.China
Dayang Chem (Hangzhou) Co.,Ltd.
P.R.China
Sancai Industry Co.,Ltd.
P.R.China
Hangzhou Keying Chem Co., Ltd.
P.R.China
Xingrui Industry Co., Limited
P.R.China
Leap Chem Co., Ltd
P.R.China
Molecular Formula: B2O3 Molecular Weight: 69.62 Hazard Symbols: Xi,T WGKGermany: 1 HS Code: 28100010
More details are to be found here
ProChem, Inc.
USA
Molecular Formula: B2O3 Molecular Weight: 69.62 Hazard Symbols: Xi,T WGKGermany: 1 HS Code: 28100010
More details are to be found here
OPQ Chemical Co., Ltd
P.R.China
Molecular Formula: B2O3 Molecular Weight: 69.62 Hazard Symbols: Xi,T WGKGermany: 1 HS Code: 28100010
More details are to be found here
Anderson & Steinssen Inc
USA
Molecular Formula: B2O3 Molecular Weight: 69.62 Hazard Symbols: Xi,T WGKGermany: 1 HS Code: 28100010
More details are to be found here
Molecular Formula: B2O3 Molecular Weight: 69.62 Hazard Symbols: Xi,T WGKGermany: 1 HS Code: 28100010
More details are to be found here
Photoelectric grade, purity 2N
Shandong Ench Chemical Co.,Ltd
P.R.China
Photoelectric grade, purity 2N
Shandong Lanhai Industry Co.,Ltd.
P.R.China
Photoelectric grade, purity 2N
Krada CPS Industry S.L
Spain
Photoelectric grade, purity 2N
Shandong Minglang Chemical Co., Ltd
P.R.China
Photoelectric grade, purity 2N
Fox Chemicals GmbH
Germany
Photoelectric grade, purity 2N
Lori Industry Co., Ltd
P.R.China
Photoelectric grade, purity 2N
Skyrun Industrial Co., Ltd.
P.R.China
Photoelectric grade, purity 2N
Shandong SanYoung Industry Co., Ltd
P.R.China
Photoelectric grade, purity 2N
Xiamen Equation Chemical Co.,Ltd
P.R.China
Photoelectric grade, purity 2N
Chemos GmbH & Co. KG
Germany
Photoelectric grade, purity 2N
Photoelectric grade, purity 2N
BuGuCh & Partners
Germany
Product name: boron trioxide
Boron oxide; boron oxide; boron trioxide; boron trioxide; boron trioxide; boron trioxide; boric acid; boric acid;
English name: Boron oxide
BORON TRIOXIDE; BORON OXIDE; BORIC ANHYDRIDE; BORIC OXIDE; BORON (III) TRI-OXIDE; BORIC ACID-ANHYDRIDE; BORIC ACID, ANHYDROUS; DI-BORON TRIOXIDE
CAS: 1303-86-2
Molecular formula: B2O3
Molecular weight: 69.62
EINECS: 215-125-8
Category: Industrial / Fine Chemicals; Inorganics; Coating Materials; Cb Boride; Inorganic Chemical Products; Inorganic Salts; Metal Oxide; Metals; Catalysis and Inorganic Chemistry; General Reagents; Boron;
Mol file: 1303-86-2.mol
Melting Point: 450 ° C (lit.)
The boiling point of 1860 ° C
Density 2.46 g / mL at 25 ° C (lit.)
Vapor density> 1 (vs air)
Flash point 1860 ° C
Form pellets
Water solubility 36 g / L (25 ēC)
Sensitivity
Merck 14, 1337
Stable. Moisture sensitive. Incompatible with water.
CAS database 1303-86-2 (CAS DataBase Reference)
NIST chemical substance information Diboron trioxide (1303-86-2)
EPA Chemical Substances Information Boron oxide (B2O3) (1303-86-2)
Solubility in water (g / 100ml) Solubility in 100 ml of water:
2.2 g / 20 ° C
Chemical properties Colorless glassy crystals or powder. The surface has a greasy feel, tasteless. Soluble in acid, ethanol, hot water, slightly soluble in cold water.
Use for the preparation of elemental boron and boron compounds, boron glass, optical glass, heat-resistant glass and glass fiber, also used as paint flame retardant and desiccant
Use as a silicate decomposition of the flux, dopants of semiconductor materials, heat-resistant glassware and paint refractory additives. Is the preparation of elemental boron and boron compounds of various raw materials. In the metallurgical industry for the production of alloy steel. Can be used as a catalyst for organic synthesis, high temperature lubricants additives and chemical reagents.
Uses Used as flux, also used in glass, enamel, boron compounds and raw materials for manufacturing color television industry as semiconductor dopants and silicate decomposition of the doping source of flux, can also be used for boron manufacturing
Use Metallurgy, silicate analysis in the determination of silica and alkali. Blowing pipe analysis. Decomposition of silicate flux.
Production method Atmospheric pressure boric acid sent to the heating kettle, heating, boric acid slowly dehydration. When the temperature rose to 107.5 ℃ into partial boric acid (HBO2), heated to 150 ~ 160 ℃ when transformed into tetraborate (H2B4O7), 650 ℃ above the melt produces a lot of foam, the final temperature will be maintained at 800 ~ 1000 ℃, Burning dehydration until the material is red and no longer bubbling. The relative density of the melt was 1.52. Then open the spinning machine for spinning, temperature control in the 700 ~ 900 ℃. And then the spinning machine on the oxidation of boron oxide wire cutting machine with a cut off, packaging, in the finished product of boron oxide. its
2H3BO3 → B2O3 + 3H2O
Vacuum method will be placed in stainless steel plate boric acid, the first oven baked for 1.5 h, then heated to 150 ℃, heated 4 h. Heating process should always flip, so that dehydration evenly. The material was then removed, cooled, pulverized, then placed in a vacuum oven, sealed, heated at 220 ° C for 1.5 h, then heated to 260 ° C, and heated for 4 h. The material was cooled, pulverized and put into tube furnace. The heating temperature was controlled at 280 ℃, dehydrated in vacuum for 4h, and the product of boron oxide was obtained. its
2H3BO3 → B2O3 + 3H2O
Category Pesticides
Toxic classification of poisoning
Acute toxicity Oral - mouse LD50: 3163 mg / kg; celiac - mouse LD50: 1868 mg / kg
Stimulation Data Skin - Rabbit 1000 mg; Eye - Rabbit 50 mg
Combustibility Hazardous characteristics Mix with calcium oxide or directly into lime milk. Heat mixture to incandescence.
Storage and transportation characteristics of the Treasury ventilation low-temperature drying; and lime separate storage
Fire extinguishing agent water, dry powder, carbon dioxide
Occupational Standard TWA 10 mg / m3 (dust); TWA 10 mg / m3
Product name: boron trioxide
Boron oxide; boron oxide; boron trioxide; boron trioxide; boron trioxide; boron trioxide; boric acid; boric acid;
English name: Boron oxide
BORON TRIOXIDE; BORON OXIDE; BORIC ANHYDRIDE; BORIC OXIDE; BORON (III) TRI-OXIDE; BORIC ACID-ANHYDRIDE; BORIC ACID, ANHYDROUS; DI-BORON TRIOXIDE
CAS: 1303-86-2
Molecular formula: B2O3
Molecular weight: 69.62
EINECS: 215-125-8
Category: Industrial / Fine Chemicals; Inorganics; Coating Materials; Cb Boride; Inorganic Chemical Products; Inorganic Salts; Metal Oxide; Metals; Catalysis and Inorganic Chemistry; General Reagents; Boron;
Mol file: 1303-86-2.mol
Melting Point: 450 ° C (lit.)
The boiling point of 1860 ° C
Density 2.46 g / mL at 25 ° C (lit.)
Vapor density> 1 (vs air)
Flash point 1860 ° C
Form pellets
Water solubility 36 g / L (25 ēC)
Sensitivity
Merck 14, 1337
Stable. Moisture sensitive. Incompatible with water.
CAS database 1303-86-2 (CAS DataBase Reference)
NIST chemical substance information Diboron trioxide (1303-86-2)
EPA Chemical Substances Information Boron oxide (B2O3) (1303-86-2)
Solubility in water (g / 100ml) Solubility in 100 ml of water:
2.2 g / 20 ° C
Chemical properties Colorless glassy crystals or powder. The surface has a greasy feel, tasteless. Soluble in acid, ethanol, hot water, slightly soluble in cold water.
Use for the preparation of elemental boron and boron compounds, boron glass, optical glass, heat-resistant glass and glass fiber, also used as paint flame retardant and desiccant
Use as a silicate decomposition of the flux, dopants of semiconductor materials, heat-resistant glassware and paint refractory additives. Is the preparation of elemental boron and boron compounds of various raw materials. In the metallurgical industry for the production of alloy steel. Can be used as a catalyst for organic synthesis, high temperature lubricants additives and chemical reagents.
Uses Used as flux, also used in glass, enamel, boron compounds and raw materials for manufacturing color television industry as semiconductor dopants and silicate decomposition of the doping source of flux, can also be used for boron manufacturing
Use Metallurgy, silicate analysis in the determination of silica and alkali. Blowing pipe analysis. Decomposition of silicate flux.
Production method Atmospheric pressure boric acid sent to the heating kettle, heating, boric acid slowly dehydration. When the temperature rose to 107.5 ℃ into partial boric acid (HBO2), heated to 150 ~ 160 ℃ when transformed into tetraborate (H2B4O7), 650 ℃ above the melt produces a lot of foam, the final temperature will be maintained at 800 ~ 1000 ℃, Burning dehydration until the material is red and no longer bubbling. The relative density of the melt was 1.52. Then open the spinning machine for spinning, temperature control in the 700 ~ 900 ℃. And then the spinning machine on the oxidation of boron oxide wire cutting machine with a cut off, packaging, in the finished product of boron oxide. its
2H3BO3 → B2O3 + 3H2O
Vacuum method will be placed in stainless steel plate boric acid, the first oven baked for 1.5 h, then heated to 150 ℃, heated 4 h. Heating process should always flip, so that dehydration evenly. The material was then removed, cooled, pulverized, then placed in a vacuum oven, sealed, heated at 220 ° C for 1.5 h, then heated to 260 ° C, and heated for 4 h. The material was cooled, pulverized and put into tube furnace. The heating temperature was controlled at 280 ℃, dehydrated in vacuum for 4h, and the product of boron oxide was obtained. its
2H3BO3 → B2O3 + 3H2O
Category Pesticides
Toxic classification of poisoning
Acute toxicity Oral - mouse LD50: 3163 mg / kg; celiac - mouse LD50: 1868 mg / kg
Stimulation Data Skin - Rabbit 1000 mg; Eye - Rabbit 50 mg
Combustibility Hazardous characteristics Mix with calcium oxide or directly into lime milk. Heat mixture to incandescence.
Storage and transportation characteristics of the Treasury ventilation low-temperature drying; and lime separate storage
Fire extinguishing agent water, dry powder, carbon dioxide
Occupational Standard TWA 10 mg / m3 (dust); TWA 10 mg / m3
BASF Belgium S.A. / N.V.
Belgium
Product name: boron trioxide
Boron oxide; boron oxide; boron trioxide; boron trioxide; boron trioxide; boron trioxide; boric acid; boric acid;
English name: Boron oxide
BORON TRIOXIDE; BORON OXIDE; BORIC ANHYDRIDE; BORIC OXIDE; BORON (III) TRI-OXIDE; BORIC ACID-ANHYDRIDE; BORIC ACID, ANHYDROUS; DI-BORON TRIOXIDE
CAS: 1303-86-2
Molecular formula: B2O3
Molecular weight: 69.62
EINECS: 215-125-8
Category: Industrial / Fine Chemicals; Inorganics; Coating Materials; Cb Boride; Inorganic Chemical Products; Inorganic Salts; Metal Oxide; Metals; Catalysis and Inorganic Chemistry; General Reagents; Boron;
Mol file: 1303-86-2.mol
Melting Point: 450 ° C (lit.)
The boiling point of 1860 ° C
Density 2.46 g / mL at 25 ° C (lit.)
Vapor density> 1 (vs air)
Flash point 1860 ° C
Form pellets
Water solubility 36 g / L (25 ēC)
Sensitivity
Merck 14, 1337
Stable. Moisture sensitive. Incompatible with water.
CAS database 1303-86-2 (CAS DataBase Reference)
NIST chemical substance information Diboron trioxide (1303-86-2)
EPA Chemical Substances Information Boron oxide (B2O3) (1303-86-2)
Solubility in water (g / 100ml) Solubility in 100 ml of water:
2.2 g / 20 ° C
Chemical properties Colorless glassy crystals or powder. The surface has a greasy feel, tasteless. Soluble in acid, ethanol, hot water, slightly soluble in cold water.
Use for the preparation of elemental boron and boron compounds, boron glass, optical glass, heat-resistant glass and glass fiber, also used as paint flame retardant and desiccant
Use as a silicate decomposition of the flux, dopants of semiconductor materials, heat-resistant glassware and paint refractory additives. Is the preparation of elemental boron and boron compounds of various raw materials. In the metallurgical industry for the production of alloy steel. Can be used as a catalyst for organic synthesis, high temperature lubricants additives and chemical reagents.
Uses Used as flux, also used in glass, enamel, boron compounds and raw materials for manufacturing color television industry as semiconductor dopants and silicate decomposition of the doping source of flux, can also be used for boron manufacturing
Use Metallurgy, silicate analysis in the determination of silica and alkali. Blowing pipe analysis. Decomposition of silicate flux.
Production method Atmospheric pressure boric acid sent to the heating kettle, heating, boric acid slowly dehydration. When the temperature rose to 107.5 ℃ into partial boric acid (HBO2), heated to 150 ~ 160 ℃ when transformed into tetraborate (H2B4O7), 650 ℃ above the melt produces a lot of foam, the final temperature will be maintained at 800 ~ 1000 ℃, Burning dehydration until the material is red and no longer bubbling. The relative density of the melt was 1.52. Then open the spinning machine for spinning, temperature control in the 700 ~ 900 ℃. And then the spinning machine on the oxidation of boron oxide wire cutting machine with a cut off, packaging, in the finished product of boron oxide. its
2H3BO3 → B2O3 + 3H2O
Vacuum method will be placed in stainless steel plate boric acid, the first oven baked for 1.5 h, then heated to 150 ℃, heated 4 h. Heating process should always flip, so that dehydration evenly. The material was then removed, cooled, pulverized, then placed in a vacuum oven, sealed, heated at 220 ° C for 1.5 h, then heated to 260 ° C, and heated for 4 h. The material was cooled, pulverized and put into tube furnace. The heating temperature was controlled at 280 ℃, dehydrated in vacuum for 4h, and the product of boron oxide was obtained. its
2H3BO3 → B2O3 + 3H2O
Category Pesticides
Toxic classification of poisoning
Acute toxicity Oral - mouse LD50: 3163 mg / kg; celiac - mouse LD50: 1868 mg / kg
Stimulation Data Skin - Rabbit 1000 mg; Eye - Rabbit 50 mg
Combustibility Hazardous characteristics Mix with calcium oxide or directly into lime milk. Heat mixture to incandescence.
Storage and transportation characteristics of the Treasury ventilation low-temperature drying; and lime separate storage
Fire extinguishing agent water, dry powder, carbon dioxide
Occupational Standard TWA 10 mg / m3 (dust); TWA 10 mg / m3
Santa Cruz Biotechnology, Inc.
USA
Product name: boron trioxide
Boron oxide; boron oxide; boron trioxide; boron trioxide; boron trioxide; boron trioxide; boric acid; boric acid;
English name: Boron oxide
BORON TRIOXIDE; BORON OXIDE; BORIC ANHYDRIDE; BORIC OXIDE; BORON (III) TRI-OXIDE; BORIC ACID-ANHYDRIDE; BORIC ACID, ANHYDROUS; DI-BORON TRIOXIDE
CAS: 1303-86-2
Molecular formula: B2O3
Molecular weight: 69.62
EINECS: 215-125-8
Category: Industrial / Fine Chemicals; Inorganics; Coating Materials; Cb Boride; Inorganic Chemical Products; Inorganic Salts; Metal Oxide; Metals; Catalysis and Inorganic Chemistry; General Reagents; Boron;
Mol file: 1303-86-2.mol
Melting Point: 450 ° C (lit.)
The boiling point of 1860 ° C
Density 2.46 g / mL at 25 ° C (lit.)
Vapor density> 1 (vs air)
Flash point 1860 ° C
Form pellets
Water solubility 36 g / L (25 ēC)
Sensitivity
Merck 14, 1337
Stable. Moisture sensitive. Incompatible with water.
CAS database 1303-86-2 (CAS DataBase Reference)
NIST chemical substance information Diboron trioxide (1303-86-2)
EPA Chemical Substances Information Boron oxide (B2O3) (1303-86-2)
Solubility in water (g / 100ml) Solubility in 100 ml of water:
2.2 g / 20 ° C
Chemical properties Colorless glassy crystals or powder. The surface has a greasy feel, tasteless. Soluble in acid, ethanol, hot water, slightly soluble in cold water.
Use for the preparation of elemental boron and boron compounds, boron glass, optical glass, heat-resistant glass and glass fiber, also used as paint flame retardant and desiccant
Use as a silicate decomposition of the flux, dopants of semiconductor materials, heat-resistant glassware and paint refractory additives. Is the preparation of elemental boron and boron compounds of various raw materials. In the metallurgical industry for the production of alloy steel. Can be used as a catalyst for organic synthesis, high temperature lubricants additives and chemical reagents.
Uses Used as flux, also used in glass, enamel, boron compounds and raw materials for manufacturing color television industry as semiconductor dopants and silicate decomposition of the doping source of flux, can also be used for boron manufacturing
Use Metallurgy, silicate analysis in the determination of silica and alkali. Blowing pipe analysis. Decomposition of silicate flux.
Production method Atmospheric pressure boric acid sent to the heating kettle, heating, boric acid slowly dehydration. When the temperature rose to 107.5 ℃ into partial boric acid (HBO2), heated to 150 ~ 160 ℃ when transformed into tetraborate (H2B4O7), 650 ℃ above the melt produces a lot of foam, the final temperature will be maintained at 800 ~ 1000 ℃, Burning dehydration until the material is red and no longer bubbling. The relative density of the melt was 1.52. Then open the spinning machine for spinning, temperature control in the 700 ~ 900 ℃. And then the spinning machine on the oxidation of boron oxide wire cutting machine with a cut off, packaging, in the finished product of boron oxide. its
2H3BO3 → B2O3 + 3H2O
Vacuum method will be placed in stainless steel plate boric acid, the first oven baked for 1.5 h, then heated to 150 ℃, heated 4 h. Heating process should always flip, so that dehydration evenly. The material was then removed, cooled, pulverized, then placed in a vacuum oven, sealed, heated at 220 ° C for 1.5 h, then heated to 260 ° C, and heated for 4 h. The material was cooled, pulverized and put into tube furnace. The heating temperature was controlled at 280 ℃, dehydrated in vacuum for 4h, and the product of boron oxide was obtained. its
2H3BO3 → B2O3 + 3H2O
Category Pesticides
Toxic classification of poisoning
Acute toxicity Oral - mouse LD50: 3163 mg / kg; celiac - mouse LD50: 1868 mg / kg
Stimulation Data Skin - Rabbit 1000 mg; Eye - Rabbit 50 mg
Combustibility Hazardous characteristics Mix with calcium oxide or directly into lime milk. Heat mixture to incandescence.
Storage and transportation characteristics of the Treasury ventilation low-temperature drying; and lime separate storage
Fire extinguishing agent water, dry powder, carbon dioxide
Occupational Standard TWA 10 mg / m3 (dust); TWA 10 mg / m3
|

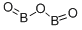
 details
details details
details details
details

_Co_Ltd_1_575.jpg)






