|
|
|

|
Suppliers for
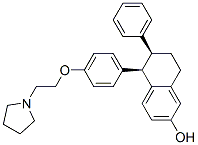
Lasofoxifene
|
Properties | | CAS |
180916-16-9 | | Formula |
C28H31NO2 |
|
|
5 Registered suppliers
Molecular Formula: C28H31NO2 Molecular Weight: 413.558
More details are to be found here
More details are to be found here
Description : Lasofoxifene (INN) (proposed tradename Fablyn) is a non-steroidal selective estrogen receptor modulator (SERM) which is under development by Pfizer for the prevention and treatment of osteoporosis and for the treatment of vaginal atrophy, and the result of an exclusive research collaboration with Ligand Pharmaceuticals (LGND). In September 2005, Pfizer received a non-approvable letter from the U.S. Food and Drug Administration regarding lasofoxifene ( Oporia), a selective estrogen receptor modulator for the prevention of osteoporosis. Lasofoxifene was approved in the EU under the Fablyn by the EMEA in March 2009. Lasofoxifene is a desmethyl dihydro analog of nafoxidine. - Molecular Weight :413.561
- Melting Point :110-113°C
- Purity :> 98%
Molecular Formula : C28H31NO2 Canonical SMILES : C1CCN(C1)CCOC2=CC=C(C=C2)C3C(CCC4=C3C=CC(=C4)O)C5=CC=CC=C5
InChI :
InChI=1S/C28H31NO2/c30-24-11-15-27-23(20-24)10-14-26(21-6-2-1-3-7-21)28(27)22-8-12-25(13-9-22)31-19-18-29-16-4-5-17-29/h1-3,6-9,11-13,15,20,26,28,30H,4-5,10,14,16-19H2/t26-,28+/m1/s1
InChIKey :
GXESHMAMLJKROZ-IAPPQJPRSA-N
Appearance :
white solid powder
Synonyms :
CP 336156; CP336156; CP-336156; CP 336,156; CP336,156; CP-33,6156; Fably; Oporia
More details are to be found here
Description : Lasofoxifene (INN) (proposed tradename Fablyn) is a non-steroidal selective estrogen receptor modulator (SERM) which is under development by Pfizer for the prevention and treatment of osteoporosis and for the treatment of vaginal atrophy, and the result of an exclusive research collaboration with Ligand Pharmaceuticals (LGND). In September 2005, Pfizer received a non-approvable letter from the U.S. Food and Drug Administration regarding lasofoxifene ( Oporia), a selective estrogen receptor modulator for the prevention of osteoporosis. Lasofoxifene was approved in the EU under the Fablyn by the EMEA in March 2009. Lasofoxifene is a desmethyl dihydro analog of nafoxidine. - Molecular Weight :413.561
- Melting Point :110-113°C
- Purity :> 98%
Molecular Formula : C28H31NO2 Canonical SMILES : C1CCN(C1)CCOC2=CC=C(C=C2)C3C(CCC4=C3C=CC(=C4)O)C5=CC=CC=C5
InChI :
InChI=1S/C28H31NO2/c30-24-11-15-27-23(20-24)10-14-26(21-6-2-1-3-7-21)28(27)22-8-12-25(13-9-22)31-19-18-29-16-4-5-17-29/h1-3,6-9,11-13,15,20,26,28,30H,4-5,10,14,16-19H2/t26-,28+/m1/s1
InChIKey :
GXESHMAMLJKROZ-IAPPQJPRSA-N
Appearance :
white solid powder
Synonyms :
CP 336156; CP336156; CP-336156; CP 336,156; CP336,156; CP-33,6156; Fably; Oporia
More details are to be found here
Description : Lasofoxifene (INN) (proposed tradename Fablyn) is a non-steroidal selective estrogen receptor modulator (SERM) which is under development by Pfizer for the prevention and treatment of osteoporosis and for the treatment of vaginal atrophy, and the result of an exclusive research collaboration with Ligand Pharmaceuticals (LGND). In September 2005, Pfizer received a non-approvable letter from the U.S. Food and Drug Administration regarding lasofoxifene ( Oporia), a selective estrogen receptor modulator for the prevention of osteoporosis. Lasofoxifene was approved in the EU under the Fablyn by the EMEA in March 2009. Lasofoxifene is a desmethyl dihydro analog of nafoxidine. - Molecular Weight :413.561
- Melting Point :110-113°C
- Purity :> 98%
Molecular Formula : C28H31NO2 Canonical SMILES : C1CCN(C1)CCOC2=CC=C(C=C2)C3C(CCC4=C3C=CC(=C4)O)C5=CC=CC=C5
InChI :
InChI=1S/C28H31NO2/c30-24-11-15-27-23(20-24)10-14-26(21-6-2-1-3-7-21)28(27)22-8-12-25(13-9-22)31-19-18-29-16-4-5-17-29/h1-3,6-9,11-13,15,20,26,28,30H,4-5,10,14,16-19H2/t26-,28+/m1/s1
InChIKey :
GXESHMAMLJKROZ-IAPPQJPRSA-N
Appearance :
white solid powder
Synonyms :
CP 336156; CP336156; CP-336156; CP 336,156; CP336,156; CP-33,6156; Fably; Oporia
More details are to be found here
|
Properties:
... more properties and specification on Lasofoxifene
|
|
Privileged suppliers
Last update 2024-04-24
|


 details
details details
details



